
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Indicate which of the following molecules is polar because it possesses a net dipole. Show the direction of the net dipole if one exists.
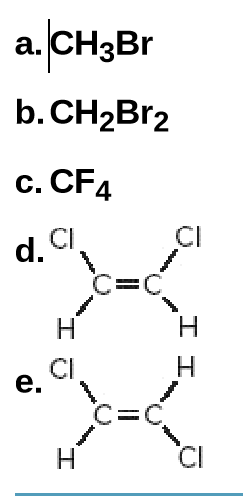
Transcribed Image Text:a.CH3B
b. CH2B12
c. CF4
d. CI
C%3C
CI
Н
CI
e.
=C.
Н
CI
エエ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the geometry about interior atom in molecule and draw the molecule. (Skeletal structure is indicated in parentheses.) NH2CO2H (H2NCOOH both O atoms attached to C)arrow_forwardWHich molecule is the most polar and has the largest NET dipole movement? NH3 NF3 PH3 PF3arrow_forwardDescribe what a dipole is and how to determine if a compound has a dipolearrow_forward
- Identify the molecular and electron geometries around each carbon (left and right). S: H H:C : C:H .. H Left Carbon Right Carbon Mol. Geo.: Mol. Geo.: Electron Geo. Electron Geo: 5.] Use electronegativity values to identify whether the following molecules are polar or nonpolar (explain why?). Indicate the polarity of each bond (towards, or from the central atom) using arrows. The molecular geometry is given in parentheses. (2 points) CH2B12 (tetrahedral geometry with C in the center) Br becavse H- Br Home Seos (trigonal planar with Se in the center) 11 4.arrow_forwardThe three-dimensional structure of a generic molecule is given. Identify the axial and equatorial atoms in the three-dimensional structure. to Answer Bank ахial equatorial Rotate X Rotate Y Rotate Z Zoom In Zoom Out Label Atoms What is the shape of this molecule? shape:arrow_forwardWhich of the following molecules is nonpolar? Group of answer choices HCN SO2 PCl3 CBr4arrow_forward
- Data table Molecule Perspective Drawing Electronegativity Bond Polarity Dipole movement Polarity BeC12 CH4 NH3 H2O SnCl2 HCN H2CO IF5arrow_forwardA molecule with nonpolar bonds can still be polar. True Falsearrow_forwardFollowing is a molecule with polar bonds whose shape was obtained using the VSEPR theory. Specify the molecular shape of this molecule, and whether the molecule is polar or nonpolar. (Hint: In terms of polarity, see whether the dipoles in the molecule cancel or not. A molecule containing polar bonds can be nanpolar if the dipoles cancel each other. You can imagine the dipoles as ropes pulling on the central atom–If the pulls cancel each other, that is, the central atom cannot move, then the molecule is nonpolar. If on the other hand the opposite is true, then the molecule is polar.) O trigonal pyramidal shape, nonpolar O trigonal planar shape, nonpolar O tetrahedral shape, polar O trigonal pyramidal shape, polar O trigonal planar shape, polararrow_forward
- 1. Briefly explain in your own words why the bond angle increases as the number of electron groups decrease? 2. How does electron domain geometry differ from molecular geometry? Briefly explain in your own words. 3. Write a complete list of steps you will utilize to predict the electron-domain geometry for a given species using only the Lewis structure. The only information you are provided with is the molecular formula and the net charge.arrow_forwardIs carbon trichloride a polar molecule?arrow_forwardWhich molecule is polar ? In image is the multiple choicesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY