
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Chem 101
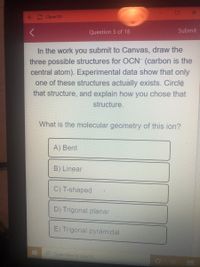
Question 5 of 18
Submit
In the work you submit to Canvas, draw the
three possible structures for OCN (carbon is the
central atom). Experimental data show that only
one of these structures actually exists. Circle
that structure, and explain how you chose that
structure.
What is the molecular geometry of this ion?
A) Bent
B) Linear
C) T-shaped
D) Trigonal planar
E) Trigonal pyramidal
Type here to searchi
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need hand written solution onlyarrow_forward- Click to edit molecule Question 15 of 46 Draw the Lewis structure of hydronium (H₂O) and then determine its electron domain and molecular geometries. A) tetrahedral / planar B) tetrahedral / trigonal pyramidal C) planar / planar D) trigonal planar / linear E) linear / linear Larrow_forwardUse Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements. Ca and Te Express your answer as a chemical formula. Mg and Br Express your answer as a chemical formula. Na and S Express your answer as a chemical formula. In and O Express your answer as a chemical formula.arrow_forward
- Following is a molecule with polar bonds whose shape was obtained using the VSEPR theory. Specify the molecular shape of this molecule, and whether the molecule is polar or nonpolar. (Hint: In terms of polarity, see whether the dipoles in the molecule cancel or not. A molecule containing polar bonds can be nanpolar if the dipoles cancel each other. You can imagine the dipoles as ropes pulling on the central atom–If the pulls cancel each other, that is, the central atom cannot move, then the molecule is nonpolar. If on the other hand the opposite is true, then the molecule is polar.) O trigonal pyramidal shape, nonpolar O trigonal planar shape, nonpolar O tetrahedral shape, polar O trigonal pyramidal shape, polar O trigonal planar shape, polararrow_forward1. Draw the best Lewis dot structure for the anion CCI in the correct molecular geometry [Include formal charges and lone pair electrons, and use dashed and solid wedge bonds if necessary 2. How many electron groups are present around the central atom and what is the electron group geometry? 3. What is the molecular geometry and ideal bond angles? 4. Is the molecule polar or nonpolar? If it is polar, draw a dipole moment arrow next to your structure to indicate the directionality of the dipole moment. Answers Edit View Insert Format Tools Table 12ptv Paragaph BIVA 2 Owordh AG3454jpg IMG 3450jpgarrow_forwardPredicting and naming the shape of molecules with a central atom.arrow_forward
- For each, draw the lewis structure, a 3D Structure including Angles, Electron Pair Geometry, Molecular Geometry, and whether it is "Polar or Non/Polar" A) SF6 B) CH2Oarrow_forwardThe shape of the water molecule (SO3) is A) linear B) tetrahedral C) trigonal pyramidal D) bent The shape of the methane molecule (NO3) is A) linear B) tetrahedral C) trigonal pyramidal D trigonal planararrow_forwardWe don't see the answer written with a photo or pen, give the answer using the toolarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY