
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
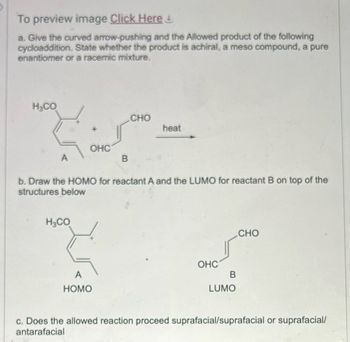
Transcribed Image Text:To preview image Click Here!
a. Give the curved arrow-pushing and the Allowed product of the following
cycloaddition. State whether the product is achiral, a meso compound, a pure
enantiomer or a racemic mixture.
H&CO
OHC
A
B
CHO
heat
b. Draw the HOMO for reactant A and the LUMO for reactant B on top of the
structures below
H₂CO
3
A
HOMO
OHC
B
LUMO
CHO
c. Does the allowed reaction proceed suprafacial/suprafacial or suprafacial/
antarafacial
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structure of the major organic product of the following reaction.arrow_forwardA. Give the curved arrow-ppushing & the allowed product of the following cycloaddition. State whether the allowed product is achiral, a meso compound, a pure eantiomer, or a racemic mixture. B. Draw the H/O/M/O for reactant A & the L/U/M/O for reactant B on top of the structures below. C. Does the allowed reaction proceed suprafacial/suprafacial or suprafacial/antarafacial.arrow_forwardNeed answer step by steparrow_forward
- Draw the product of each SN2 reaction and indicate stereochemistry. CH3CH₂ a. H C-Br + OCH₂CH3 b. + -CNarrow_forwardSelect the major product for the following reactions Br₂ EtOH A. B. C. D. E. Me e H H + enantiomer Br.. Ber: JBr + enantiomer Asal Br +enantiomer Eto... enantiomer QEt + enantiomerarrow_forwardA generic solid, X, has a molar mass of 77.577.5 g/mol. In a constant‑pressure calorimeter, 23.323.3 g of X is dissolved in 297297 g of water at 23.00 °C. X(s)⟶X(aq) The temperature of the resulting solution rises to 29.5029.50 °C. Assume the solution has the same specific heat as water, 4.184 J/(g·°C), and that there is negligible heat loss to the surroundings. How much heat was absorbed by the solution? q= kJ What is the enthalpy of the reaction? Δ?rxn=. kJ/molarrow_forward
- Draw it outarrow_forwardHCCH In this reaction, which species acts as the electrophile? H3C HọC-N-B-H Học® H CH3 H H;C CHy B. H H CC O a. CCL O b. H;C H H;C-N-B-H H;C® H Oc. CH3 Hoc CH3 O d H .B.arrow_forward2. When the molecule shown below is heated up in water, two alcohols that are constitutional isomers are formed. Predict the structures for the two alcohols. Which do you suppose would be major? Why? Show the mechanism for the reaction you are proposing. Is your mechanism an SN1, SN2, E1, or E2 pathway? H c- Br Which major? alcohol A Why major? mechanism: Mechanism pathway: SN1 or alcohol B SN2 El alcohol A alcohol B Are your alcohols A and B constitutional isomers? Y N E2arrow_forward
- R.arrow_forward6. Determine whether the following reactions are SN1 or SN2. Draw the mechanism and the products with stereochemistry. a. b. Br Br + "CN + -OCH 3 acetone DMSO d. e. f. Br 11 + CH3OH + CH3CO₂H Dº Br + -OCH₂CH3 + CH3CH₂OH DMF 1, 2 of 2arrow_forwardDraw tructural or or he ajor roduct f he eaction shown. CH3CH=CHCH3 Cl2 • Do not show stereóchemist in other cases. • If the reaction produces a racemic mixture, just draw one stereoisomer. • Show product stereochem IF the reactant alkene has both carbons of the double bond within a ring. C. opy P. C. progress ChemDoodle (Previous Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY