
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Predict the geometry around each highlighted atom.
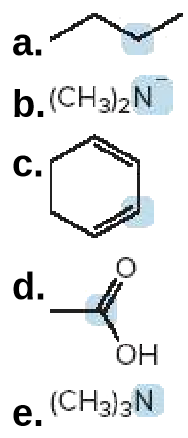
Transcribed Image Text:a.
b.(CH3)2N
C.
d.
OH
e. (CH3)3N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Add lone pairs to the central atoms as necessary to complete the Lewis structures. Do not worry about the positions of the lone pairs on the central atom. Focus on the number of lone pairs. XEF2 SF, Erase Select Draw Rings More Erase Select Draw Rings More S F Xe Xe F :arrow_forward9.arrow_forwardWhen pure sulfuric acid is dissolved in water, heat is evolved. 10.65 g of pure sulfuric acid at 20.00 °C was added to a calorimeter containing 300 g of water at 20.00 °C. The temperature of the solution increased to 26.35 °C. If the specific heat of the mixture is 4.184 Jg1°C1, the density of water is 1 g/mL, and the heat capacity of the calorimeter is ignored, what is the heat evolved per mole of sulfuric acid? a. -83.2 kJ O b. -73.6 kJ O C. -76.0 kJ d. -80.8 kJ e. -78.4 kJarrow_forward
- what is the geometry around the double bonds ? from left to rightarrow_forwardDraw all 6 constitutional isomers of C3H6O each containing one double bond (one pibond). Draw each in Lewis structure and bondline format (zigzag structures )arrow_forwarde. Select the name of the following molecular structure. Obent O tetrahedral see-saw trigonal bipyramid Otrigonal pyramidarrow_forward
- Which drawing best represents the resonance hybrid for the given molecule? S* Ď 8*arrow_forwardWhat’s the total number of VSEPR groups in HCN?arrow_forwardplease check the screenshot fiind Lewis Structure (show all electrons and bonds) e– geometry Approx. Bond angles Polar or non-polar (don't worry about ions) Total lone pairs in the entire molecule Number of nonbonding electron pairs on the central atomarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY