
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Need help with HW packet question 3,4,5
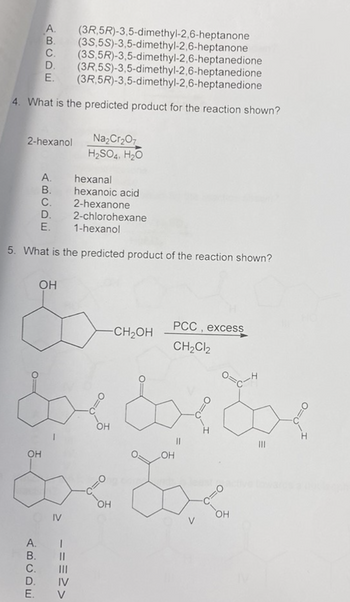
Transcribed Image Text:A. (3R,5R)-3,5-dimethyl-2,6-heptanone
B. (35,5S)-3,5-dimethyl-2,6-heptanone
C. (3S,5R)-3,5-dimethyl-2,6-heptanedione
(3R,5S)-3,5-dimethyl-2,6-heptanedione
(3R,5R)-3,5-dimethyl-2,6-heptanedione
D.
E.
4. What is the predicted product for the reaction shown?
2-hexanol Na₂Cr₂O7
H₂SO4, H₂O
OH
ABCDE
5. What is the predicted product of the reaction shown?
ABCDE
A.
B.
OH
&
A.
B.
E.
E.
dxdxdx
LOH
hexanal
hexanoic acid
2-hexanone
2-chlorohexane
1-hexanol
IV
===>>
OH
-CH₂OH
OH
PCC, excess
CH₂Cl2
V
OH
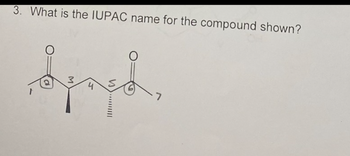
Transcribed Image Text:3. What is the IUPAC name for the compound shown?
dened,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How do I solve for [FeSCN2+] heres the formula they provided me and my data but I would like steps on how to solve it and what data I should be using to solve for it u can use any fake numbers you need to help me understand ! I'm confused only by the absorbance part , what number am I using for the absorbance? Is it Fe3+ added to ScN- absorbance values ? also e = 202 and L = 1.0arrow_forwardHelp needed with assigning the peak signals and determining the J values of this Isoamyl Acetate molecule. Thank you so much!arrow_forwardLOD TRANSMITTANCE1% D 4000 O ketone Oester 3000 2000 O carboxylic acid O aldehyde O alcohol O nitrile (i.e., RCN) O None of these other choices is correct. HAVENUMBERI-I 1500 1000 500arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY