
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:% TRANSMITTANCE
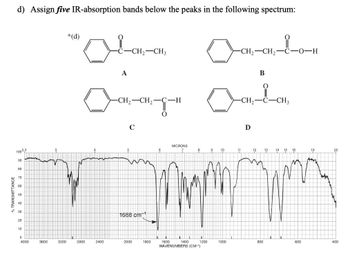
d) Assign five IR-absorption bands below the peaks in the following spectrum:
*(d)
A
CH2-CH3
-CH2-CH2-C-H
C
Ñcot;Ñct;ཡརི་ཡo-t
B
cut.ཡ་ཡct;
Ꭰ
10025
90
60
50
40
30
20
8 2 2 2 2 2 2 2 2
10
1688 cm
MICRONS
8
9
10
11
12 13
14
15 16
19
0
4000
3600
3200
2800
2400
2000
1800
1600
1400
1200
1000
800
600
400
WAVENUMBERS (CM-1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Fill out the IR spectra of the compoundarrow_forwardidentify and interpret the peaks and their characteristics (C=O, 4H, doublet) product name: dulcin (4-ethoxyphenylurea) (C9H12N2O2) images include CNMR, HNMR, and IR spectroscopyarrow_forward[X] (ppm) 0.00 10.0 15.0 20.0 25.0 30.0 35.0 x-axis Resources UV-Vis absorbance data for molecule X collected at 425 nm using a 3.50 cm cuvet is given in the table. [X] (M) 0.00 1.00 × 10 4 1.50 × 10-4 2.00 × 10-4 2.50 × 10-4 3.00 x 10-4 3.50 x 10-4 O %T %T T A 100.0 1.000 0.000 49.4 0.494 0.306 34.9 0.349 0.457 25.3 0.253 0.597 17.3 0.173 0.761 15.4 0.154 0.813 13.9 0.139 0.857 Prepare a plot using the linear range of the data to determine the molar absorptivity. What should be plotted on each axis to determine the molar absorptivity of molecule X at this wavelength? x Give Up? y-axis Hint OT 8:1arrow_forward
- Find the structural shape of the compound according to the infrared spectrum?arrow_forward% Transmittance 180 90 80 70 60 50 40 30 20 10 0- 4000 5 H 8 (c) Below is a IR, ¹H NMR and ¹³C NMR spectra of an unknown compound. Draw the structure of the compound. Molecular Formula = C9H10O2 ¹H NMR spectrum 7 C-H stretch (aromatic) 160 140 3078 6 2986 C-H stretch (alkyl) 5 C=O stretch 1726 2000 Wavenumbers (cm-1) 2 H 4 ppm 120 100 PPM 3 80 13 2 3 H 60 1117 C-O stretches 1286 1 C NMR Spectrum 0 40 20 12arrow_forwardCould you please interpret this? I know it is a very weak alcohol, but I’m unsure beyond that.arrow_forward
- A very simple IR spectrum of a drug does not contain bands above 3000 not any significant bands in the 1500-1700 range. Using your tables, decide which of these compounds satisfy these spectral characteristics 0000 B в ОН C ОН Darrow_forwardInterpet and analyze this Infrared (IR) spectra to show the signs from the peaks and what they tell about the unknown compound.arrow_forwardWhich of the shown compounds corresponds to the shown IR spectrum ? Micrometers 2.5 100 8 10 12 13 14 15 20 90 80 70 50 40 30 20 10 4000 3600 3200 2800 2400 2000 1800 1600 1400 1200 1000 800 600 400 Wavenumber (cm-!) NH2 Transmittance (%)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY