
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
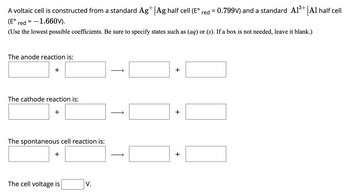
Transcribed Image Text:A voltaic cell is constructed from a standard Ag+ Ag half cell (E° red = 0.799V) and a standard A1³+ Al half cell
(E° red=-1.660V).
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.)
The anode reaction is:
+
The cathode reaction is:
+
The spontaneous cell reaction is:
+
The cell voltage is
V.
1
↑
↑
+
+
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A voltaic cell is constructed from a standard Cu2+ | Cu half cell (E° red = .337V) and a standard Br2 | Br- half cell (E° red = 1.080V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The spontaneous cell reaction is: + + The cell voltage is V.arrow_forwardA voltaic cell is constructed from a standard Cr³+1 Cr half cell (E° red = -0.740V) and a standard Fe³+ | Fe2+ half cell (Eºred = 0.771V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: + The spontaneous cell reaction is: The cell voltage is V. + + + [arrow_forwardA voltaic cell is constructed from a standard Cu²+ Cu half cell (E° red 0.337V) and a standard Br2 |Br half cell (E° 1.080V). red (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. + + ↑arrow_forward
- A voltaic cell is constructed in which the anode is a Pb|Pb2+ half cell and the cathode is a I-|I2 half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The net cell reaction is: + + In the external circuit, electrons migrate _(from or to?)__ the I-|I2 electrode _(from or to?)__the Pb|Pb2+ electrode.In the salt bridge, anions migrate _(from or to?)__ the Pb|Pb2+ compartment _(from or to?)__ the I-|I2 compartment.arrow_forward2+ 2+ A voltaic cell is constructed from a standard Sn²+ Sn half cell (E° red = -0.140V) and a standard Fe³+ Fe²+ half cell (E° red = 0.771V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. ↑ 1 ↑ + + +arrow_forwardI dont know how to do this questionarrow_forward
- A voltaic cell is constructed from a standard = Sn2+ Sn half cell (E° red -0.140V) and a standard F2 F half cell (E° red = 2.870V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. ↑ + + +arrow_forwardA voltaic cell is constructed from a standard Ag+ | Ag half cell (E° red = .799V) and a standard Br2 | Br- half cell (E° red = 1.080V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The spontaneous cell reaction is: + + The cell voltage is V.arrow_forwardA voltaic cell is constructed from a standard Hg2* Hg half cell (E°red = 0.855V) and a standard Br2 Br half cell (E°red = 1.080V). (Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: The spontaneous cell reaction is: The cell voltage is V.arrow_forward
- A voltaic cell is constructed from a standard Cr+ Cr half cell (E°red = -0.740V) and a standard F2F half cell (E°. red = 2.870V). (Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.) The anode reaction is: per) Greek(lower) Arrows Other The cathode reaction is: + elp The spontaneous cell reaction is: The cell voltage is V.arrow_forwardA lithium battery is being developed. Its voltaic cell is constructed based on Li metal electrode and PbsO4 solid electrode. Their standard reduction potentials at 25 °C are given below. E°(Li* (aq) → Li (s)) = -3.040 Volt E°(PbSO4 (S) > Pb (s) + SO42 (aq) = - 0.356 Volt. Which of the following statements is true about this working (spontaneous) voltaic cell? a. The anode is PbsO4 (s) electrode. The standard cell potential of this voltaic cell is E°(cell) = 2.328 Volt O. PBSO4 (s) is a weaker oxidizing agent than Lit. To make a commercial product, the voltaic cell is adjusted to have [Lit] = 2.1 M and [SO42] = 3.5 M. The cell d. potential E under this condition should be less than the standard cell potential E°(cell). e. None of the abovearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY