
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
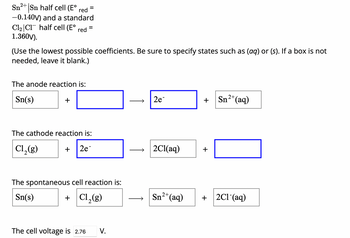
Transcribed Image Text:Sn2+ Sn half cell (E° red =
-0.140V) and a standard
C12 C1 half cell (E° red =
1.360V).
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not
needed, leave it blank.)
The anode reaction is:
Sn(s)
+
The cathode reaction is:
Cl2(g)
+
2e-
The spontaneous cell reaction is:
Sn(s)
+ Cl2(g)
The cell voltage is 2.76 V.
2e
+
Sn2+(aq)
2Cl(aq)
+
Sn2+(aq)
+ 2C1-(aq)
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- [References] Consider the galvanic cell based on the following half-reactions: Zn2+ + 2e Zn e =-0.76 V Fet + 2e+ Fe e = -0.44 V a. Determine the overall cell reaction and calculate e (Use the lowest possible coefficients. Be sure to specify states such as (ag) or (s). If a box is not needed, leave it blank.) E ell b. Calculate AG° and K for the cell reaction at 25°C. AG 6 69x10 10 k] K = C. Calculate Ecelt at 25°C when Zn-= 0.10 M and Fe²| = 8.0 × 10-0 M . Ecell =0 199arrow_forwardConsider the galvanic cell based on the following half-reactions. Ag+ + e− → Ag ℰ° = +0.80 V Fe2+ + 2 e− → Fe ℰ° = −0.44 V (a) Determine the overall cell reaction and calculate ℰ ° cell . (b) Calculate ΔG° and K for the cell reaction at 25°C. (c) Calculate ℰcell at 25°C when [Ag+ ] = 1.0 ✕ 10−4 M and [Fe2+ ] = 1.0 ✕ 10−2 M.arrow_forwardA voltaic cell is constructed from a standard Cu2+ | Cu half cell (E° red = .337V) and a standard Br2 | Br- half cell (E° red = 1.080V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The spontaneous cell reaction is: + + The cell voltage is V.arrow_forward
- A voltaic cell is constructed from a standard Cr³+1 Cr half cell (E° red = -0.740V) and a standard Fe³+ | Fe2+ half cell (Eºred = 0.771V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: + The spontaneous cell reaction is: The cell voltage is V. + + + [arrow_forwardA voltaic cell is constructed from a standard Cu²+ Cu half cell (E° red 0.337V) and a standard Br2 |Br half cell (E° 1.080V). red (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. + + ↑arrow_forwardConsider the galvanic cell based on the following half-reactions: Zn+ + 2e + Zn e° = -0.76 V Cd²+ + 2e → Cd e° = -0.40 V a. Determine the overall cell reaction and calculate e (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (5). If a box is not needed, leave it blank.) b. Calculate AG° and K for the cell reaction at 25°C. AG kJ K = [Zn**] = 0.10 M and [Ca] = c. Calculate e cell at 25°C when Zn 1.2 x 10-5 M. Ecell =arrow_forward
- A voltaic cell is constructed in which the anode is a Pb|Pb2+ half cell and the cathode is a I-|I2 half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The net cell reaction is: + + In the external circuit, electrons migrate _(from or to?)__ the I-|I2 electrode _(from or to?)__the Pb|Pb2+ electrode.In the salt bridge, anions migrate _(from or to?)__ the Pb|Pb2+ compartment _(from or to?)__ the I-|I2 compartment.arrow_forwardA voltaic cell is constructed from a standard Cd2+|Cd half cell (E°red = -0.403V) and a standard Br2|Br- half cell (E°red = 1.080V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The spontaneous cell reaction is: + + The cell voltage is V.arrow_forward2+ 2+ A voltaic cell is constructed from a standard Sn²+ Sn half cell (E° red = -0.140V) and a standard Fe³+ Fe²+ half cell (E° red = 0.771V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. ↑ 1 ↑ + + +arrow_forward
- 2. In the electrolysis of an aqueous Zn(NO3)2 solution, what reactions occur at the anode and at the cathode, assuming standard conditions? O, + 4H + 4e → 2H;O 6° = 1.23 V 2H,0 + 2e → H2 + 20H E° = -0.83V Zn2 + 2e → Zn ɛ° = -0.76 V NO, + 4H + 3e' → NO + 2H20 E° = 0,96 V ANODE: CATHODE:arrow_forwardA voltaic cell is constructed from a standard = Sn2+ Sn half cell (E° red -0.140V) and a standard F2 F half cell (E° red = 2.870V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. ↑ + + +arrow_forwardA voltaic cell is constructed from a standard Ag+ | Ag half cell (E° red = .799V) and a standard Br2 | Br- half cell (E° red = 1.080V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) The anode reaction is: + + The cathode reaction is: + + The spontaneous cell reaction is: + + The cell voltage is V.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY