
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
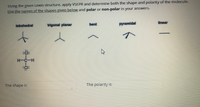
Transcribed Image Text:Using the given Lewis structure, apply VSEPR and determine both the shape and polarity of the molecule.
Use the names of the shapes given below and polar or non-polar in your answers.
linear
tetrahedral
trigonal planar
bent
pyramidal
:Ci:
H-C-H
The shape is
The polarity is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Predicting whether molecules are polar or nonpolar 3/5 Da Decide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion polar or nonpolar? atom closest to negative side O polar NH3 O nonpolar O polar OcS O nonpolar O polar N2 O nonpolar Explanation Check 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility II IIarrow_forwardWhat is the % by mass of water in your hydrated copper (Il) sulphate? Mass of just anhydrous compound = mass of anhydrous compound - mass of beaker and rod 108.34g - 106.87g = 1.47g %D %3D = original compound mass - anhydrous compound mass = 3.00g - 1.47g = 1.53g Mass of just water %Darrow_forwardWhat is the bond angle of a tetrahedral molecule?arrow_forward
- Apply VSEPR concepts and draw the shapes of the following molecules. Also indicate whether they are polar or nonpolar. The central atoms are shown in boldface. HF CO2 N2 H2S Polar or nonpolar? Polar or nonpolar? Polar or nonpolar? Polar or nonpolar? Circle one Circle one Circle one Circle onearrow_forwardUse the following information to determine the Lewis structure, find the electron and molecular geometry of the molecule, determine the angle of the molecule, and determine the polarity. If the atoms are not the same, you may assume that the difference in their electronegativities are between 0.4 and 2.0. Atom information: A: 6 valence electrons, CAN exceed the octet. Further from fluorine on the periodic table than X. X: 6 valence electrons, CANNOT exceed the octet. Closer to fluorine on the periodic table than A. Molecule: AX2 Electron Geometry: [Select 1 Molecular Geometry: I Select ] Bond Angle: [ Select] Polarity: [ Select]arrow_forwardUse the following information to determine the Lewis structure, find the electron and molecular geometry of the molecule, determine the angle of the molecule, and determine the polarity. If the atoms are not the same, you may assume that the difference in their electronegativities are between 0.4 and 2.0. Atom information: A: 8 valence electrons, CAN exceed the octet. Further from fluorine on the periodic table than X. X: 7 valence electrons, CANNOT exceed the octet. Closer to fluorine on the periodic table than A. Molecule: AX4 Electron Geometry: [Select] Molecular Geometry: [Select] Bond Angle: [ Select ] Polarity: [ Select ]arrow_forward
- Decide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table, molecule or polyatomic ion polar or nonpolar? atom closest to negative side O polar CH, CI O nonpolar O polar co, O nonpolar O polar CIF O nonpolar 回 国arrow_forwardUsing the image attached: What is the electron geometry of this molecule? What is the molecular geometry (shape) of this molecule? Is this molecule polar or non-polar? Explain how you determined the polar/non-polar nature of this molecule.arrow_forwardDecide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. atom closest to negative side molecule or polar or попрolar? polyatomic lon O polar O nonpolar O polar CH,F O nonpolar O polar O nonpolar H,Sarrow_forward
- Draw the structure for O2 and then complete the following sentences regarding its structure. This molecule has a total of and a total of a pairs of bonding electrons pairs of nonbonding electrons. This molecule has shape. The polarity of this molecule isarrow_forwardAnswer the questions in the table below about the shape of the periodate (10) anion. How many electron groups are around the central iodine atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central iodine atom? (You may need to use the scrollbar to see all the choices.) Continue 787 11 FILMADON FALCION POGING FEB 27 0 (choose one) linear bent T-shaped trigonal planar trigonal pyramidal square planar square pyramidal tetrahedral sawhorse trigonal bipyramidal octahedral 3 4 tv Ⓒ2023 M Sarrow_forwardHow to get the molecular geometry of a molecule and bond angles?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY