
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
review the section on Bond Polarity and Partial Ionic Character and the figure that has ionic character on the Y axis and delta EN on the X axis (this is figure 9.24 on page 393 of the 8th edition).
Review the graph and Critique the following statement: HF is a covalent molecule and LiI is an ionic compound. Provide as much detail and explanation as possible in 100 word or less. As stated previously, equations, formulas etc. may be substituted for words.
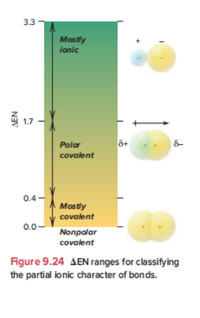
Transcribed Image Text:**Figure 9.24** - ΔEN Ranges for Classifying the Partial Ionic Character of Bonds
This figure illustrates how the difference in electronegativity (ΔEN) between two bonded atoms can be used to classify the type of bond based on its ionic character. The vertical gradient bar is labeled with different ΔEN values and associated bond types:
- ΔEN from 0.0 to 0.4: **Nonpolar covalent** bonds
- ΔEN from 0.4 to 1.7: **Mostly covalent** bonds
- ΔEN from 1.7 to 3.3: **Polar covalent** bonds
- ΔEN above 3.3: **Mostly ionic** bonds
On the right side of the gradient bar, there are illustrations showing the distribution of electron density in these types of bonds:
1. **Nonpolar covalent**: Shown with two identical atoms sharing electrons equally. The electron cloud is symmetrically distributed.
2. **Polar covalent**: Depicted with one atom partially positive (δ+) and the other partially negative (δ-), indicating uneven electron distribution.
3. **Mostly ionic**: The electron cloud is shown significantly shifted toward the atom with higher electronegativity, forming a clear positive and negative side.
This diagram helps in understanding how electronegativity differences affect bond character, ranging from equal sharing (nonpolar covalent) to complete transfer of electrons (mostly ionic).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please send the answer by typing only. I don't want handwritten.arrow_forward1 What is the total number of valence electrons in the Lewis structure of BrO4"? electrons 2 Draw a Lewis structure for BrO4 • Do not include overall ion charges or formal charges in your drawing. • If the species contains oxygen, do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. 0- Y **** O. Sn [1 2. Draw Lewis Structures (Octet and Nonoctet): This is group attempt 1 of 5arrow_forwardPlease help me with this question (Explain). What would be the correct asnwers?arrow_forward
- the halogens, X2 Draw the molecule by placing atoms on the grid and connecting them with bonds. Include nonbonding electrons. Use X to indicate a halogen atom.arrow_forwardWhich of the following bonds are classified as being a polar bond. (Show your calculations and explain your answer.) N-N O-H Cl-H N-Farrow_forwardConsider the following Lewis symbols for elements X and Y: X. and. Y. You may want to reference (Pages 185-188) Section 6.5 while completing this problem. Q Search ▼ Part E vetemme now any ions are needed for a total ionic 6- charge. What would be the formula of a compound of X and nitrogen? Express your answer as a chemical formula. ΑΣΦ Review TUIs dit needed LU UDIdill d Uldi IVIC UT a Submit Request Answer Part F Complete previous part(s) A chemical reaction does not occur for this question. S ?arrow_forward
- Question 12 of 21 View Policies Show Attempt History Current Attempt in Progress. Use Lewis symbols to diagram the formation of Cal2 from Ca and I atoms. Ca2+ 2|:: | Ca: 2|:1:| Ca2+ + Ca2+ 2|::| Ca: Ca* + 2[: | eTextbook and Media Hint P Type here to search O aarrow_forwardFollowing is a molecule with polar bonds whose shape was obtained using the VSEPR theory. Specify the molecular shape of this molecule, and whether the molecule is polar or nonpolar. (Hint: In terms of polarity, see whether the dipoles in the molecule cancel or not. A molecule containing polar bonds can be nanpolar if the dipoles cancel each other. You can imagine the dipoles as ropes pulling on the central atom–If the pulls cancel each other, that is, the central atom cannot move, then the molecule is nonpolar. If on the other hand the opposite is true, then the molecule is polar.) O trigonal pyramidal shape, nonpolar O trigonal planar shape, nonpolar O tetrahedral shape, polar O trigonal pyramidal shape, polar O trigonal planar shape, polararrow_forwardI do not understand itarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY