
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
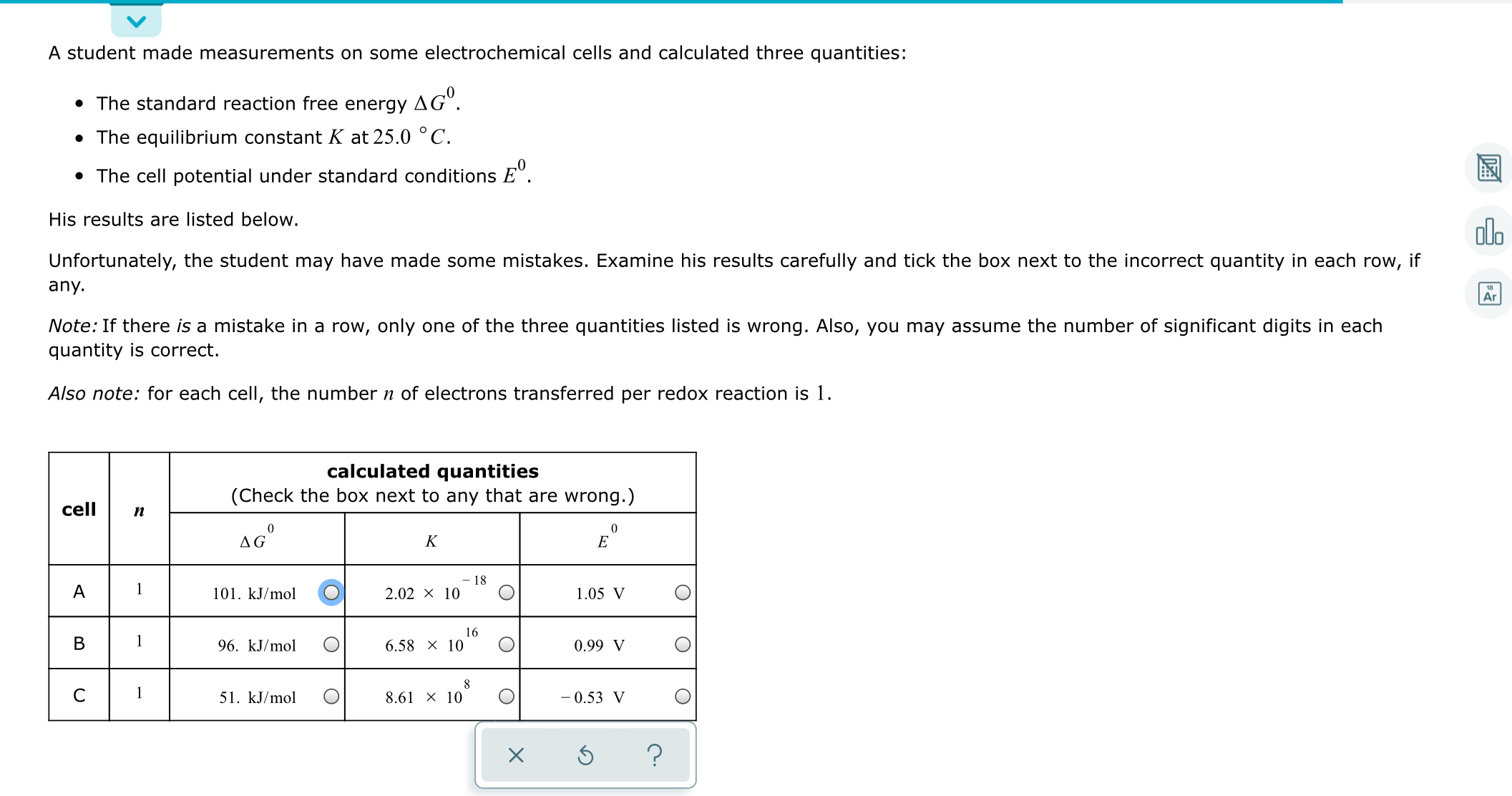
Transcribed Image Text:A student made measurements on some electrochemical cells and calculated three quantities:
• The standard reaction free energy
до".
• The equilibrium constant K at 25.0 ° C.
• The cell potential under standard conditions E°.
His results are listed below.
ola
Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row,
if
any.
Ar
Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each
quantity is correct.
Also note: for each cell, the number n of electrons transferred per redox reaction is 1.
calculated quantities
(Check the box next to any that are wrong.)
cell
п
AG°
K
18
1
101. kJ/mol
2.02 × 10
1.05 V
16
1
96. kJ/mol
6.58 x 10
0.99 V
1
51. kJ/mol
8.61 x 10
- 0.53 V
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Determine the standard free energy change, Gf, for the formation of S2-(aq) given that the Gf for. Ag*(aq) and Ag2S(s) are 77.1 kJ/mole and 395 kJ/mole respectively, and the solubility product for Ag2S(s) is 81051.arrow_forwardThe overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 K is 2H2(g)+O2(g)2H2O(l)K=1.281083 a. Calculate and G at 298 K for the fuel cell reaction. b. Predict the signs of H and S for the fuel cell reaction. c. As temperature increases, does the maximum amount of work obtained from the fuel cell reaction increase, decrease, or remain the same? Explain.arrow_forwardFor each of the following reactions, determine the overall balanced electrochemical reaction, its standard electric potential, and the standard Gibbs energy of the reaction. aCo+F2Co2++2F bZn+Fe2+Zn2++Fe c Zn+Fe3+Zn2++Fe d Hg2++HgHg22+arrow_forward
- For each of the reactions, calculate E from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard conditions. (a) Cu2+(aq)+Ni(s)Cu(s)+Ni2+(aq) (b) 2Ag(s)+Cl2(g)2AgCl(s) (c) Cl2(g)+2I(aq)2Cl(aq)+I2(s)arrow_forwarda Calculate G for the following cell reaction: Tl(s)Tl+(aq)Pb2+(aq)Pb(s) The Gf for Tl+(aq) is 32.4 kJ/mol. b From G, calculate the standard cell potential for the cell reaction and from this, determine the standard potential for Tl2+(aq)+eTl(s).arrow_forwardConsider the reaction low at 25°C: 3SO42(aq)+12H+(aq)+2Cr(s)3SO2(g)+2Cr3+(aq)+6H2O Use Table 17.1 to answer the following questions. Support your answers with calculations. (a) Is the reaction spontaneous at standard conditions? (b) Is the reaction spontaneous at a pH of 3.00 with all other ionic species at 0.100 M and all gases at 1.00 atm? (c) Is the reaction spontaneous at a pH of 8.00 with all other ionic species at 0.100 M and all gases at 1.00 atm? (d) At what pH is the reaction at equilibrium with all other ionic species at 0.100 M and all gases at 1.00 atm?arrow_forward
- Consider the reaction below at 25°C: 2MnO4(aq)+16H+(aq)+10Br(aq)2Mn2+(aq)+5Br2(l)+8H2O Use Table 17.1 to answer the following questions. Support your answers with calculations. (a) Is the reaction spontaneous at standard conditions? (b) Is the reaction spontaneous at a pH of 2.00 with all other ionic species at 0.100 M? (c) Is the reaction spontaneous at a pH of 5.00 with all other ionic species at 0.100 M? (d) At what pH is the reaction at equilibrium with all other ionic species at 0.100 M?arrow_forwardWhen magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is oxidized and that hydrogen is reduced, write the balanced equation for the reaction. How many electrons are transferred in the balanced equation? What quantity of useful work can be obtained when Mg is added directly to the beaker of HCl? How can you harness this reaction to do useful work?arrow_forwardCalculate E°, G°, and K at 25°C for the reaction 3Mn2+(aq)+2MnO4(aq)+2H2O5MnO2(s)+4H+(aq)arrow_forward
- Calculate E°, G°, and K at 25°C for the reaction 3MnO4(aq)+4H+(aq)+Cl2(g)2Mn2+(aq)+2ClO3(aq)+2H2Oarrow_forwardCalculate G at 355 K for each of the reactions in Question 17. State whether the reactions are spontaneous.arrow_forwardCalculate K at 25°C for each of the reactions referred to in Question 32. Assume smallest whole-number coefficients.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning