
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
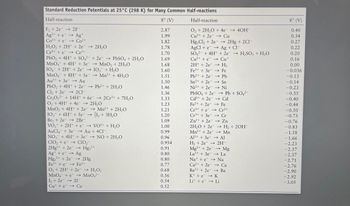
Transcribed Image Text:Standard Reduction Potentials at 25°C (298 K) for Many Common Half-reactions
Half-reaction
8° (V)
Half-reaction
F₂ +2e2F-
Ag2+ + e
Ag+
Co³+ + e→→ Co²+
H₂O₂ + 2H+ + 2e
Ce4++e Ce³+
→ 2H₂O
PbO₂ + 4H+ + SO42
MnO4 + 4H+ + 3e
IO4 + 2H+ + 2e →
MnO4 + 8H+ + Se
Au³+ + 3e → Au
PbO₂ + 4H+ + 2e
Cl₂ +2e2C1-
Hg₂2+ + 2e
Fe³+ + e
+2e →→ PbSO4 + 2H₂O
→ MnO₂ + 2H₂O
IO3 + H₂O
→ Mn²+ + 4H₂O
→ Pb²+ + 2H₂O
Cr₂O72- + 14H+ + 6e- →2Cr³+ + 7H₂O
O₂ + 4H+ + 4e → 2H₂O
MnO₂ + 4H+ + 2e
IO3 + 6H+ + Se-
Br₂ 2e 2Br
VO₂+ + 2H+ + e
AuCl4 + 3e →
NO3 + 4H+
ClO₂ + e
2Hg2+ + 2e
Age → Ag
→ Mn²+ + 2H₂O
→ 1₂ + 3H₂O
→ VO2+ + H₂O
Au + 4CI-
+ 3e → NO + 2H₂O
ClO₂-
→ Hg₂²+
→ 2Hg
Fe2+
O₂ + 2H+ + 2e → H₂O₂
MnO4 + e→→ MnO4²-
1₂ +2e → 21-
Cute
Cu
2.87
1.99
1.82
1.78
1.70
1.69
1.68
1.60
1.51
1.50
1.46
1.36
1.33
1.23
1.21
1.20
1.09
1.00
0.99
0.96
0.954
0.91
0.80
0.80
0.77
0.68
0.56
0.54
0.52
O₂ + 2H₂O + 4e4OH-
Cu2+ + 2e → Cu
Hg₂Cl₂ + 2e →→ 2Hg + 2Cl-
AgCl +eAg + Cl-
SO4 + 4H+ + 2e
Cu²+ + e
2H+ + 2e
Fe³+ + 3e
Cu+
→→ H₂
→→ Fe
→→ Pb
→→ Sn
→→ Ni
Pb2+ + 2e
Sn²+ + 2e
Ni2+ + 2e
PbSO4 + 2e
Cd2+ + 2e
→→ Pb + SO4²-
→→ Cd
→→→ Fe
Fe2+ + 2e
Cr³+ + e
Cr²+
Cr³+ + 3e
→ Cr
→ Zn
Zn²+ + 2e
2H₂O + 2e
→ H₂ + 2OH-
Mn²+ + 2e → Mn
Al³+ + 3e →→ Al
H₂ +2e → 2H-
Mg2+ + 2e → Mg
La³+ + 3e → La
→→→ Na
Na+ + e
Ca2+ + 2e
Ba2+ + 2e
K++eK
Lite Li
→ H₂SO3 + H₂O
→→ Ca
→→ Ba
8° (V)
0.40
0.34
0.27
0.22
0.20
0.16
0.00
-0.036
-0.13
-0.14
-0.23
-0.35
-0.40
-0.44
-0.50
-0.73
-0.76
-0.83
-1.18
-1.66
-2.23
-2.37
-2.37
-2.71
-2.76
-2.90
-2.92
-3.05
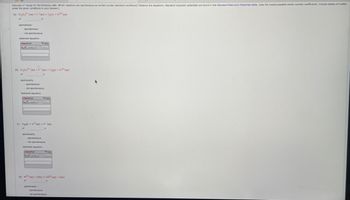
Transcribed Image Text:Calculate values for the following cells. Which reactions are spontaneous as written (under standard conditions)? Balance the equations. Standard reduction potentials are found in the Standard Reduction Potentials table. (Use the lowest possible whole number coefficients. Include states-of-matter
under the given conditions in your answer.)
(a) Cr₂0₂²(aq) + 1(aq) = 1₂(s) + Cr³+ (aq)
spontaneity:
spontaneous
not spontaneous
balanced equation:
chemPad
(b) Cr₂072 (aq) + F(aq) = F₂(g) + Cr³+ (aq)
f
Help
De
spontaneity:
spontaneous
not spontaneous
balanced equation:
chemPad
spontaneity:
(c) H₂(g) H(aq) + H(aq)
spontaneous
not spontaneous
balanced equation
chemPad
spontaneity:
(d) Ni2+ (aq) + Ca(s) - Cd²+ (aq) + Ni(s)
V
spontaneous
Help
not spontaneous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- With explanation Find the standard change in free energy and the equilibrium constant for the galvanic cell resulting from the cell notation provided below. P(s)|Cr2+(aq),Cr3+(aq)|| Pb2+(aq)|Pb(s)arrow_forwardBalance the following reaction. Calculate the cell potential and the Gibbs free energy of the reaction at standard conditions. Write the line representations for the Galvanic cells, indicating the anode and cathode.arrow_forwardConstruct a galvanic cell from MnO4- (aq)/MnO2 (s) and H2 O2 (aq)/H2 O (l).a) Write a balanced equation for the cell.b) What is the value of E°cell? standard Ni (s) | Ni2+ (aq) || Ag+ (aq) | Ag (s) is constructed and the standard cell potential is1.03 V.a) Write a balanced reaction for the cell.b) What is ∆G° for the cell?arrow_forward
- Calculate the standard cell potential, ?∘cellEcell°, for the reaction shown. Use these standard reduction potentials. Cu(s)+Ag+(aq)⟶Cu+(aq)+Ag(s)arrow_forwardCalculate the standard cell potential for the following galvanic cell. Ni(s)Ni(NO3)>(aq)||A9NO3(aq)|Ag(s)arrow_forward3. Calculate the standard potentials for each of the cells at 298 K. Is each combination spontaneous as written? (a) Ag (s) | Ag + (aq) || Au *3 (aq) | Au (s) (b) Cu (s) | Cu +2 (aq) || Al *3 (aq) | Al (s)arrow_forward
- Write a balanced equation for the overall cell reaction in the following galvanic cell and tell why inert electrodes are required at the anode and cathode. Pt(s) | Br (aq) | Br₂ (1) || Cl; (g) | Cl(aq) | Pt(s)arrow_forwardA chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential 2+ Zn (aq)+2e - Zn(s) :-0.763 V (red |Cro (aq)+4 H2O(1)+3e Cr(OH)3(s)+5OH (aq) :-0.13 V = - (red Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. x10 Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written.arrow_forwardBalance the following reactions. Calculate the cell potential and the Gibbs free energy of these reactions at standard conditions. Identify the reactions with K > 1 among the following redox reactions. Write the line representations for the Galvanic cells, indicating the anode and cathode.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY