
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
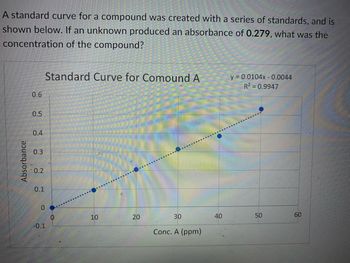
Transcribed Image Text:A standard curve for a compound was created with a series of standards, and is
shown below. If an unknown produced an absorbance of 0.279, what was the
concentration of the compound?
Absorbance
0.6
0.5
0.4
0.3
0.2
0.1
Standard Curve for Comound A
0
-0.1
0
10
20
30
Conc. A (ppm)
40
y = 0.0104x -0.0044
R² = 0.9947
50
60
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given this data, if a CV solution has an absorbance of 1.125, what is [CV]? [CV] / M A 1.0 × 10–6 M 0.250 3.0 × 10–6 M 0.750 Question 12 options: 4.5 × 10–6 M 5.0 × 10–6 M 5.5 × 10–6 Marrow_forwardSubstance P has an extinction coefficient of 72.22 mM-1cm-1 at 420nm. 2 μl of Substance P was added to 998 μl water and the Absorbance at 420nm was 0.820. Calculate the mM concentration of the original Substance P solutionarrow_forwardThe molar absorptivity constant of a particular chemical is 2.31 M/cm. Determine the concentration of a solution made from this chemical that has an absorbance of 0.408 with a cell path length of 1.12 cm.arrow_forward
- em1.png Part 3: Q9 Average absorbance Flask 8 Flask 9 Flask 10 1:1 Ratio so, Flask 11 Part 3: Q Flask 8 Flask 9 Flask 10 Flask 11 Part 3: Q10 Flask 8 Flask 9 Flask 10 Flask 11 Part 3 0.146 0.166 [Fe] -0.002mol/L 0.215 0.294 [FESCN³mol/L) 4.59% 10 5.18*10* 6.64*10* 9.08*10* [FXmol/L) 5.00 *10* 5.00*10* Y=3362x-0.0083 R² = 0.9993 5.00*10* 5.00*10* (Amounts of flask 8 (9 - 11's values are in the spreadsheet) 71% volume 5.00mL Molarity of Fe(NO3))3 = 0.2M Volume of Fe(NO3))3 = 5.00mL Moles of Fe³= Molarity x volume(L) Moles of Fe¹ = 0.2M X 0.005L = 1x10-³mole x [SCN][mol/ 4x10 5×10-4 7x10 1×10 1 Concentration of Fe³ = mole of Fe³ Volume mole of Fe³1x10³ mole G.O05L 8, Average absorbance for solution 8 (same for 9 - 11) Average absorbance for flask 8 = sum of all absorbances / number of readings (0.145 +0.147 +0.148) 3 Using equation of calibration curve's line(slope) of best fit, the average absorbances of the unknown concentrations to determine [FeSCN-Jat equilibrium in solution 8:…arrow_forwardHow do I find the theoretical molarity?arrow_forwardChemistryarrow_forward
- Suppose that a solution has an absorbance of 0.250 at a wavelength of 450 nm. If the concentration of the solution is 22 μM, what is the value of the molar absorptivity? The data were taken with a standard 1-cm cuvette. molar absorptivity in (L cm^−1 mol^-1):arrow_forwardA standard containing 20 mg/mL of compound P is placed in a 1 cm cuvette. The absorbance of the standard is 1.20 at 600 nm. A sample containing Compound P has an absorbance of 0.50. What is the concentration of this sample?arrow_forwardA medical lab technician creates a standard solutions of percent hemoglobin in whole blood. The technician measures the absorbance of each solution at 520 nm and creates a standard curve of the absorbance versus percent hemoglobin in whole blood and finds the linear equation below, with an R2 of 0.9985. y = 0.0523 x + 0.072 A patient sample has an absorbance of 0.162. Using the standard curve data, calculate the percent hemoglobin in the patient samplearrow_forward
- How would I do 2? the concentration iron is 1.85mg/vitamin tablet, and the absorbance at 508nm is 0.37arrow_forwardA colored ion solution has a concentration of 0.200 M with a measured absorbance A = 0.880. Another ion solution made of the same chemicals has an absorbance A = 0.172. What is the concentration of this unknown sample solution?arrow_forwardCan you help me on questions 2 AND 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY