
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
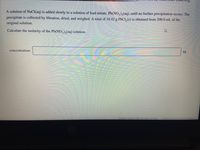
Transcribed Image Text:A solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO,),(aq), until no further precipitation occurs. The
precipitate is collected by filtration, dried, and weighed. A total of 18.42 g PbCI, (s) is obtained from 200.0 mL of the
original solution.
Calculate the molarity of the Pb(NO,),(aq) solution.
concentration:
Question Source: MRG - General Chemistry
Publisher: University Science Books
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO3)2(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 12.40 g PbCl2(s) is obtained from 200.0 mL200.0 mL of the original solution. Calculate the molarity of the Pb(NO3)2(aq) solution.arrow_forwardcopper (II) ion is precipitated from solution by adding 3.00 M NaOH(aq) resulting in a blue precipitate. What volume in milliliters would be necessary to fully precipitate the copper (II) ion from 100.0 mL of a 0.288 M solution of copper (II) nitrate.arrow_forwardA solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO,),(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 19.74 g PbCl, (s) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb(NO,),(aq) solution. concentration: Marrow_forward
- A solution was made by adding water to 0.293 g of H2ZH2Z until the volume totaled 25.00 mL. Subsequent titration required 40.50 mL of 0.093 M sodium hydroxide. Calculate the molar mass of H2ZH2Z (in g/mol).arrow_forwardwhen 100.0 mL of a 2.00 M solution of KOH(aq) is added to 100.0 mL of a 2.00 M solution of Mg(NO3)2 what is the mass of the precipitate(s) that form?arrow_forwardSolid potassium cyanide is slowly added to 75.0 mL of a 0.360 M nickel(II) acetate solution until the concentration of cyanide ion is 0.0231 M. The mass of nickel ion remaining in solution is gramsarrow_forward
- A solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO,),(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 13.88 g PbCl, (s) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb(NO,),(aq) solution. concentration: M & TOOLS x10 Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry | Publisher: University Science Booksarrow_forwardThe balanced equation for the reaction of aqueous Pb(CIO,), with aqueous NaI is Pb(CIO,),(aq) + 2 Nal(aq) → Pbl,(s) + 2 NaCIO, (aq) What mass of precipitate will form if 1.50 L of concentrated Pb(CIO,), is mixed with 0.650 L of 0.250 M Nal? Assume the reaction goes to completion. mass of precipitate:arrow_forwardBy titration, it is found that 66.5 mL of 0.158 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCl solution.arrow_forward
- An excess of sodium carbonate solution is added to 75.0 ml of calcium chloride solution. 7.50 g precipitate is formed. Calculate the concentration of the calcium chloride solution.arrow_forwardV A chemistry student weighs out 0.135 g of phosphoric acid (H₂PO4), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1200M NaOH solution. O CHEMICAL REACTIONS Determining the volume of base needed to titrate a given mass... Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits. icrosoft O W Welcome, Simrat - Microsoft tab os lock 0 Microsoft 6.52.210... esc mL control Explanation 1 Q A 2 option Z Check @ 2 W S #3 X command x10 X E D NOV 14 $ 4 C S R 8 F 07 dº % 5 T V tv MacBook Pro < 6 G Y & 7 H Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility U * 00 8 B N J "A ( 9 M K O W O L H P command 33 + 11 [ I optarrow_forwardSolid potassium sulfide is slowly added to 150 mL of a 0.374 M cobalt(II) bromide solution until the concentration of sulfide ion is 0.0660 M. The mass of cobalt(II) ion remaining in solution isarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY