
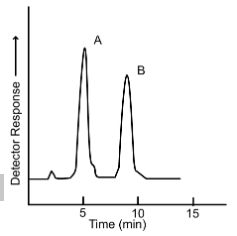
a sample of butyl benzoate is prepared to use as an internal standard. The results of a preliminary run, which used a solution known to contain 1.53 mg/mL of methyl benzoate (peak A) and1.68 mg/mL of butyl benzoate (peak B), are shown. The area of peak A is determined to be 352 and the area of peak B is determined to be 416 measured in arbitrary units by the computer.
To measure the sample, 1.00 mL of a standard sample of butyl benzoate containing 2.19 mg/mL is mixed with 1.00 mL of the plant stream material. Analysis of the mixture gave a peak area of 449 for peak A and 411 for peak B.
What is the concentration of methyl benzoate in the plant stream?
concentration of methyl benzoate = ____________________ mg/mL
Step by stepSolved in 6 steps with 6 images

- A three-component mixture containing compounds A, B, and C is separated by simple distillation. A fraction from that distillation is collected and analyzed by gas chromatography. The resulting chromatogram is overlaid on a ruler in the figure shown below. Using this figure and the data contained therein, determine the percentage composition of each component in this fraction by the triangulation method (i.e. determine the percentages of A, B, and C in this fraction). Be sure to show the setup for your calculations. 15 10 AAA 5. B 10 15 20 25 30 35 (both axes are in mm)arrow_forwardA student would like to prepare a 0.010 M solution from a 1.0 M stock solution with minimal error. The student can choose to do a one-step dilution that uses a 10 mL transfer pipet and a 1000 mL volumetric flask or a two-step dilution using a 50 mL pipet and a 1000 mL volumetric flask for the first dilution, and a 100 mL pipet and a 500 mL volumetric flask for the second dilution. Which method has the smallest overall relative uncertainty and what is the value? **Use table 4.2 on page 70 in your textbook for tolerances of the glassware. O One-step dilution; 0.0020 Two-step dilution; 0.0020 O Two-step dilution; 0.0014 O One-step dilution; 0.0010arrow_forwardComplete the equation for the reaction between the following Lewis acid-base pair. Use curved arrows to show the flow of electrons in the reaction and draw the product. Assign lone pairs and radical electrons where appropriate. Apply formal charges where appropriate. • Draw the appropriate electron-flow arrows. • Use the "starting points" menu to revert to the original molecule(s) shown. • Omit + signs between structures. ● / CH3 1- H₂C-C CH3 H در St ? ChemDoodleⓇarrow_forward
- In this experiment you will use 5.20 g of Ph3P+CH2CO2Et Br–. How many millimoles is this?arrow_forwardA stock solution of 0.225 +/- 0.003 M NaNO2 was transferred to a 100 mL volumetric flask (class A) and diluted to the mark. If 7 mL of the stock solution was transferred using one 5-mL and two 1-mL volumetric pipettes, what is the new concentration of the solution and the uncertainty?arrow_forwardA compound “X” is regulated by health Canada at 2 ug/L in drinking water (maximum allowable concentration). What will be the minimum cut-off level of your analytical method to analyze that compound “X”? How do you determine the cut-off level of your method?arrow_forward
- Q6. The following set of millimolarities were obtained when standardizing a solution: 10.67, 10.71, 10.66, and 10.45. One value appears suspect. Using Q test determine if it can be ascribed to accidental error, at the 90% confidence level.arrow_forwardYou are analyzing the effluent from a lotion plant for parabens using HPLC. You have a working standard solution with a concentration of 59.4 ppm which returned a Peak Height of 10,164 and Peak Area of 5,315. You take 44.4 L of the water sample and concentrate it to 0.3 L and analyze this solution. The Peak Height is 9,119 and the Peak Area is 6,320. What is the concentration of parabens in the effluent of the plant in mg/L to two decimal places.arrow_forward1. A wastewater sample has the following composition: NO3 = 37 mg/L as N CO3²-2.5 x 10 mol/L PO4³ = 10 meq/L Ca²+ = 75 mg/L as CaCO3(s) Express each of the above concentrations in mg/L, mol/L, meq (of charge)/L, and ppm. Make and list any necessary assumptions. Show your work and summarize the results in a table.arrow_forward
- Three labs were contracted to measure the concentration of a 5.50 ppm Ca2* standard solution. They each measured the solution 3 times, and their results are as follows: Measurement 1 Measurement 2 Measurement 3 Laboratory 1 5.47 ppm 5.54 ppm 5.50 ppm Laboratory 2 5.32 ppm 5.44 ppm 5.56 ppm Laboratory 3 5.61 ppm 5.62 ppm 5.60 ppm Select ALL of the TRUE statements about the measurement capabilities of these labs. Lab #3 was the most precise. Lab #1 was the most precise. Lab #1 was the most accurate. Lab #2 was the most precise. O.Lab #2 was the most accurate. Lab #3 was the most accurate.arrow_forward00 mL of a diprotic acid primary standard solution was accurately prepared to a concentration of 0.1431 M. Three samples of this primary standard solution were used as samples in a titration to standardize an aqueous solution of sodium hydroxide, NaOH, which would be used as a titrant. Using the following table of data for the titration of the primary standard acid with NaOH, calculate the average concentration of NaOH. Trial # Volume of primary standard Initial titrant volume Final titrant volume 1 10.00 mL 8.21 mL 27.22 mL 2 10.00 mL 27.22 mL 46.23 mL 3 10.00 mL 30.28 mL 49.29 mL 0.1506 M 0.0753 M 0.0376 M 0.1431 M 0.0526 Marrow_forwardWhich is correct? Random errors affect accuracy and systematic errors affect precision. An accurate result shows agreement with the true or accepted value. Precise results can contain significant random error, but little systematic error. The standard deviation is a good measure of accuracy. Polydimethylsiloxane (PDMS) stationary phases have low polarities. Which of the following statements is TRUE about gas chromatography using PDMS stationary phases? The gas chromatography column is typically packed with silica microparticles. An oven is used to control the column temperature because a constant temperature is always needed in gas chromatography Octane (C8H18, boiling point: 126 ºC) is eluted later than dodecane (C12H26, boiling point: 216 ºC) 3-Pentanone ((C2H5)2CO, boiling point: 102 ºC) is eluted later than propanol (CH3CH2CH2OH, boiling point: 97 ºC)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





