
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
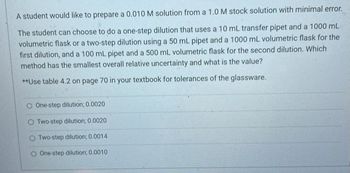
Transcribed Image Text:A student would like to prepare a 0.010 M solution from a 1.0 M stock solution with minimal error.
The student can choose to do a one-step dilution that uses a 10 mL transfer pipet and a 1000 mL
volumetric flask or a two-step dilution using a 50 mL pipet and a 1000 mL volumetric flask for the
first dilution, and a 100 mL pipet and a 500 mL volumetric flask for the second dilution. Which
method has the smallest overall relative uncertainty and what is the value?
**Use table 4.2 on page 70 in your textbook for tolerances of the glassware.
O One-step dilution; 0.0020
Two-step dilution; 0.0020
O Two-step dilution; 0.0014
O One-step dilution; 0.0010
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A series of iron standard solutions need to be made from a 40 ppm iron stock solution provided. For the first dilution, pipet 5.00 mL of the 40 ppm stock solution into a 100.00 mL volumetric flask and dilute to mark. What is the new concentration of the diluted solution?arrow_forwardPlease help me with this one Sugar is the solute and water is the solvent. The drink mix will be considered negligible and is added simply for the visualization of dilution results. Calculate the concentration of sugar in this solution using the volume percent equation. Refer to the background for sample calculations. For the purpose of this exercise, it can be assumed that the weight of the drink mix is 0.00 g. The volume of the solution is 3 and 1/3 cups. Convert to metric units, then calculate and record the volume percent in Data Table 1 (Note that 1 cup = 236 mL). Volume % = Volume of Solute/ Volume of Solution × 100%arrow_forwardStarting from the primary standard solution in Question 4 and using the dilution scheme in Figure 1, calculate the concentrations of Standards #1-5. Show all work on a piece of scratch paper. Label each calculation clearly (Standard #1, Standard #2, etc) and circle your final answer for each standard. Be sure to round your answers to the correct number of significant figures and include units. Molarity in #4 is 0.0004089 M or 4.089x10^-4arrow_forward
- 4) did i do this right?arrow_forwardA student needs to measure out ~17mL of a solution to add to a reaction container, select all pieces of equipment that can be used to accomplish this task: Group of answer choices a 20-mL beaker a 50-mL beaker a 25-mL Erlenmeyer flask a 10-mL graduated cylinder a 25-mL graduated cylinder a volumetric flask a volumetric pipetarrow_forward5.00 mL of stock solution is diluted to 25.00 mL, producing solution ALPHA. 10.00 mL of solution ALPHA is diluted to 25.00 mL, resulting in solution BETA. 10.00 mL of solution BETA is then diluted to 25.00 mL, producing solution GAMMA. dilution factor for ALPHA from stock solution = 0.167 dilution factor for BETA from ALPHA solution = 0.0476 part c and d?arrow_forward
- If you wanted to create a 2 mM solution of sodium chloride in 1000 mL from a 200 mM sodium chloride stock solution, what volume of the solution would you use? (hint: M1V1 = M2V2).arrow_forwardMake sure answer has correct significant digitsarrow_forward||| Microsoft O W OCHEMICAL REACTIONS Dilution A chemist must prepare 500. mL of 1.00 M aqueous silver perchlorate (AgCIO4) working solution. She'll do this by pouring out some 1.94 silver perchlorate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the silver perchlorate stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits. 00 mL Microsoft Microsoft 6.52.210... Explanation Check 9,180 ☐ X 285 S C Q NOV 10 ||| tv SO17 A mol L ( aqueous 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility P eBook W 1 0arrow_forward
- A chemistry student is given 1.00 L of a clear aqueous solution at 17 degrees Celsius. He is told an unknown amount of a certain compound X is dissolved in the solution. The student allows the solution to cool to 17 degrees Celsius. The solution remains clear. He then evaporates all of the water under vacuum. A precipitate remains. The student washes, dries and weighs the precipitate. It weighs 0.12 kg. Using only the information above, can you calculate the solubility of X in water at 17° C? If you said yes, calculate it and be sure your answer has a unit symbol and 2 significant digits.arrow_forwardOCHEMICAL REACTIONS Dilution A chemist must prepare 875. ml. of 335. M aqueous copper (11) fluoride (CuF) working solution. She'll do this by pouring out some 407. µμM aqueous copper (11) fluoride stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in ml. of the copper (11) fluoride stock solution that the chemist should pour out. Round your answer to 3 significant digits. 0 04 0/5 Yarrow_forwardYou measure 15.0 mL of the H2C2O4 solution with a 100-mL graduated cylinder and add it to a 125-mL Erlenmeyer flask. Then you add two drops of the indicator, phenolphthalein, to the oxalic acid solution in the flask. You dispense the NaOH solution from the buret into the flask (while swirling the flask) until you reach the endpoint. At the endpoint, the solution in the flask turns light pink. You repeat the titration two additional times for a total of three trials. H2C2O4 (aq) + 2 NaOH (aq) --> Na2C2O4 (aq) + 2 H2O (l) (I) (II) (III) Volume of H2C2O4 used 15.00 mL 15.00 mL 15.00 mL how many Moles H2C2O4 are used in each trial?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY