
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
2,6
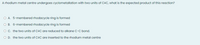
Transcribed Image Text:A rhodium metal centre undergoes cyclometallation with two units of C=C, what is the expected product of this reaction?
O A. 5-membered rhodacycle ring is formed
O B. 6-membered rhodacycle ring is formed
O C. the two units of C=C are reduced to alkane C-C bond.
O D. the two units of C=C are inserted to the rhodium metal centre
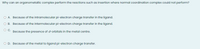
Transcribed Image Text:Why can an organometallic complex perform the reactions such as insertion where normal coordination complex could not perform?
O A. Because of the intramolecular pi-electron charge transfer in the ligand.
OB.
Because of the intermolecular pi-electron charge transfer in the ligand.
Because the presence of dorbitals in the metal centre.
O D. Because of the metal to ligand pi-electron charge transfer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statement(s) is( are) true? a. The coordination number of a metal ion in an octahedral complex ion is 8. b. All tetrahedral complex ions are low-spin. c. The formula for triaquatrianuninechromiwn(III) sulfate is [Cr(H2O)3(NH3)](SO4)3. d. The electron configuration of Hf2+ is [Xe]4f126s2. e. Hemoglobin contains Fe3+.arrow_forwarda. In the absorption spectrum of the complex ion Cr(NCS)63, there is a band corresponding to the absorption of a photon of light with an energy of 1.75 104 cm-1. Given 1 cm1 = 1.986 1023 J, what is the wavelength of this photon? b. The CrNC bond angle in Cr(NCS)63 is predicted to be 180. What is the hybridization of the N atom in the Ncs- ligand when a Lewis acid-base reaction occurs between Cr3+ and NCs- that would give a 180 CrNC bond angle? Cr(NCS)63 undergoes substitution by ethylenediamine (en) according to the equation Cr(NCS)63+2enCr(NCS)2(en)2++4NCS Does Cr(NCS)2(en)2+ exhibit geometric isomerism? Does Cr(NCS)2(en)2+ exhibit optical isomerism?arrow_forwardThe square-planar complex Pt(en)Cl2 has chloride ligands in a cis configuration. No trans isomer is known. Based on the bond lengths and bond angles of carbon and nitrogen in the ethylenediamine ligand, explain why the trans compound is not possible.arrow_forward
- The transition metals form a class of compounds called metal carbonyls, an example of which is the tetrahedral complex Ni(CO)4. Given the following thermodynamic data (at 298 K): (a) Calculate the equilibrium constant for the formation of Ni(CO)4(g) from nickel metal and CO gas. (b) Is the reaction of Ni(s) and CO(g) product- or reactant-favored at equilibrium? (c) Is the reaction more or less product-favored at higher temperatures? How could this reaction be used in the purification of nickel metal?arrow_forwardIs it possible for a complex of a metal in the transition series to have six unpaired electrons? Explain.arrow_forwardHow many geometric isomers of the complex ion [Cr(dmen)3]3+ can exist? (dmen is the bidentate ligand 1,1-dimethylethylenediamine.) 1,1-Dimethylethylenediamine, dmenarrow_forward
- Chelating ligands often form more stable complex ions than the corresponding monodentate ligands with the same donor atoms. For example, Ni2+(aq)+6NH3(aq)Ni(NH3)62+(aq)K=3.2108Ni2+(aq)+3en(aq)Ni(en)32+(aq)K=1.61018Ni2+(aq)+penten(aq)Ni(penten)2+(aq)K=2.01019 where en is ethylenediamine and penten is This increased stability is called the chelate effect. Based on bond energies, would you expect the enthalpy changes for the above reactions to be very different? What is the order (from least favorable to most favorable) of the entropy changes for the above reactions? How do the values of the formation constants correlate with S? How can this be used to explain the chelate effect?arrow_forwardTrimethylphosphine, P(CH3)3, can act as a ligand by donating the lone pair of electrons on the phosphorus atom. If trimethylphosphine is added to a solution of nickel(Il) chloride in acetone, a blue compound that has a molecular mass of approximately 270 g and contains 21.5% Ni, 26.0% Cl, and 52.5% P(CH3)3 can be isolated. This blue compound does not have any isomeric forms. What are the geometry and molecular formula of the blue compound?arrow_forwardWhat is the coordination number of the central metal atom in the following complexes? (a) [Fe(H2O)63+] (b) [Pt(NH3)Br3] (c) [V(en)Cl42] (d) [Au(CN)2+]arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning