
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
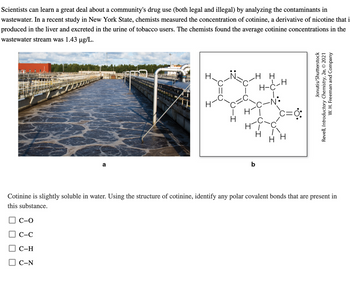
Transcribed Image Text:Scientists can learn a great deal about a community's drug use (both legal and illegal) by analyzing the contaminants in
wastewater. In a recent study in New York State, chemists measured the concentration of cotinine, a derivative of nicotine that i
produced in the liver and excreted in the urine of tobacco users. The chemists found the average cotinine concentrations in the
wastewater stream was 1.43 µg/L.
a
Ι
Ι
H-
b
HH
H
H-C
C-N:
C=0:
TH
HH
Jonutis/Shutterstock
Revell, Introductory Chemistry, 2e, © 2021
W. H. Freeman and Company
Cotinine is slightly soluble in water. Using the structure of cotinine, identify any polar covalent bonds that are present in
this substance.
C-O
C-C
C-H
C-N
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- If 42.1 mL of 0.214 M HCl solution is needed to neutralize a solution of Ca(OH)2, how many grams of Ca(OH)2 must be in the solution? Express the mass in grams to three significant digits. —| ΑΣΦ m = ? 80 garrow_forwardA chemist needs to create a series of standard Cu+(aq) solutions for an absorbance experiment. For the first standard, he uses a pipet to transfer 10.00 mL of a 2.86 M Cu(aq) stock solution to a 250.0 mL volumetric flask, and he adds enough water to dilute to the mark. He then uses a second pipet to transfer 25.00 mL of the second solution to a 50.00 mL volumetric flask, and he adds enough water to dilute to the mark. Calculate the concentration of the Cu2+(aq) solution in the 50.00 mL volumetric flask. concentration:arrow_forwardA buret is filled with 0.1517 M NaOH(aq).NaOH(aq). A 25.0 mL portion of an unknown acid HA(aq)HA(aq) and two drops of indicator are added to an Erlenmeyer flask, and the titration experiment is carried out. If the initial buret reading was 0.55 mL, and the buret reading at the end point was 22.50 mL, what is the molarity of the unknown acid, HA?arrow_forward
- Alcohol levels can be determined by reaction with dichromate (breathalyzer). C2H5OH(l) + 2 Cr2O72-(aq) + 16 H+(aq) → 4 Cr3+(aq) + 11 H2O(l) + 2 CO2(g) What is the alcohol level expressed in parts per thousand of a blood plasma if a 32.6 g sample of plasma requires 21.27 mL of a 0.02226 M solution of K2Cr2O7 for complete reaction?arrow_forwardWrite a procedure that a laboratory technician could use to prepare 2.0 L of 0.25 mol/L sulfuric acid, H2S04(aq), using 12.0 mol/L stock solution. Include appropriate safety precautions. Hypochlorous acid, HOCl(aq), is a weak acid that is used as a bleach and a disinfectant. When 25.00 mL samples of hypochlorous acid were titrated with 1.00 mol/L sodium hydroxide, NaOH(aq), an average of 19.15 mL of sodium hydroxide was needed to neutralize the acid. Calculate the molar concentration of the hypochlorous acid.arrow_forwardThere is a legend according to which a young man presented his beloved with a ring made of iron. Iron was obtained from the blood of a young man.a) Estimate how long it took the young man to excrete the required amount of iron from his blood. The boy's body weight is 80 kg. Blood makes up 8% of a person's weight, the density of blood is 1.050 g / ml, 100 g of blood contains 14 g of hemoglobin. The molar mass of hemoglobin is 68kg / mol (take into account the number of iron atoms in all subunits!).According to the rules for donating blood for men, it is safe to donate blood no more than 4-5 times a year (for calculations, use 4 times). During one collection, 450 ml is taken.b) To isolate iron from the blood, the blood was first centrifuged to separate hemoglobin, thenthe protein was destroyed with acid (that is, you have a solution containing iron cations).Suggest a further chain of reactions to obtain metallic iron. (write the reaction equations) take the mass of the ring 3 garrow_forward
- A chemist needs to create a series of standard Cu²+ (aq) solutions for an absorbance experiment. For the first standard, he uses a pipet to transfer 10.00 mL of a 2.07 M Cu²+ (aq) stock solution to a 500.0 mL volumetric flask, and he adds enough water to dilute to the mark. He then uses a second pipet to transfer 5.00 mL of the second solution to a 100.0 mL volumetric flask, and he adds enough water to dilute to the mark. Calculate the concentration of the Cu²+ (aq) solution in the 100.0 mL volumetric flask. concentration: Marrow_forwardAn aqueous solution contains 2.8 mM of total ions. Part A If the solution is NaCl(aq), what is the chloride ion concentration? Express your answer as a concentration to two significant figures. ΑΣΦ E (CI ] in NaCl(aq) = mM CI (aq) %3Darrow_forwardA chemist prepares a solution of iron II bromide FeBr2 by measuring out 17.mg of FeBr2 into a 50.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br− anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.arrow_forward
- A chemist prepares a solution of silver(I) nitrate (AgNO,) by measuring out 245. µmol of silver(I) nitrate into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in umol/L of the chemist's silver(I) nitrate solution. Be sure your answer has the correct number of significant digits. u mol x10arrow_forward0.644 g of wood bleach, which contains oxalic acid, H2C2O4, was placed in an Erlenmeyer flask. The wood bleach was titrated with 23.57 mL of 0.379 M KOH solution. Determine the percent by mass of the H2C2O4 (a diprotic acid) in the sample of wood bleach. Enter your answer with the correct number of significant figures. If you do not enter the correct number of significant figures, your answer may be graded as incorrect even if it is otherwise correct.arrow_forwardA chemist prepares a solution of vanadium(III) bromide (VBr) by measuring out 0.10 g of VBr3 into a 100. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to 2 significant digits. mol L x10 X Śarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY