
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
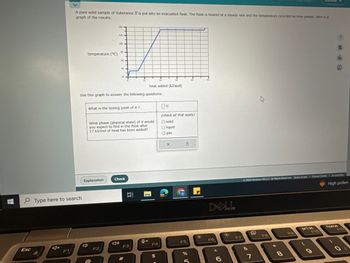
Transcribed Image Text:**Title: Understanding Heat and Phase Changes of a Pure Substance**
**Introduction:**
A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate, and the temperature recorded as time passes. The graph below illustrates the results of this experiment.
**Graph Analysis:**
- **Graph Details:**
- **X-axis (Horizontal):** Heat added (kJ/mol)
- **Y-axis (Vertical):** Temperature (°C)
- **Graph Description:**
- The graph shows three distinct segments:
1. The temperature remains constant initially as heat is added (0 to 10 kJ/mol), indicating a phase change.
2. The temperature then rises steadily (10 to 30 kJ/mol), showing heating in a single phase (liquid).
3. It levels off again at a higher temperature, marking another phase change (30 to 40 kJ/mol).
**Questions:**
1. **What is the boiling point of X?**
- Answer choices provided: Enter the temperature in °C.
2. **What phase (physical state) of X would you expect to find in the flask after 17 kJ/mol of heat has been added?**
- Answer choices:
- Solid
- Liquid
- Gas
**Instructions:**
Use the graph to deduce when these phase changes occur and accurately answer the questions above. Click ‘Check’ to verify your answers.
**Conclusion:**
This experiment helps in understanding how a substance transitions between solid, liquid, and gas phases with the application of heat, by analyzing temperature changes plotted against heat added.
**Resources:**
- For further reading and detailed explanations, please refer to our educational resources section.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the information provided below to calculate the quantity of heat (in kJ) required to convert 52.55 g of substance A from an initial temperature of -7.048°C to a final temperature of 129.6°C. The molar mass of the substance is 25.28 g/mol. Melting Point of A = 3.459°C Heat of fusion = 10.39 kJ/mol %3D %3D Boiling point of A = 107.3°C Heat of vaporization = 72.43 kJ/mol Solid Liquid Gas Specific heat (J/g°C) 2.05 4.74 6.52 Report your answer to the tenths place and do not include units.arrow_forwardA pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. A graph of the results is shown in the picture. A. Using the graph, what is the melting point of X? B. What phase (physical state) of X would you expect to find in the flask after 9 kJ/mol of heat has been added? Select all that apply: solid, liquid, or gasarrow_forwardWhich phase has a fixed shape? Solid O Gas O Liquidarrow_forward
- I'm not sure how I could answer this question.arrow_forwardThe temperature on a sample of pure X held at 1.13 atm and -9. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.39 atm. On the phase diagram below draw a path that shows this set of changes. 200 400 temperature (K) pressure (atm)arrow_forwardpure sou sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a aph of the results: temperature (°C) 200. 180,- 160.- 120.- 100.- 10. What is the melting point of X ? heat added (kJ/mol) Use this graph to answer the following questions: What phase (physical state) of X would you expect to find in the flask after 7 kJ/mol of heat has been added? 0°C (check all that apply) solid O liquid Ogas 1arrow_forward
- need help with this chemistryarrow_forwardA pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: 110. 90. temperature (°C) 70. 50. 30. 0. 10. 20. 30. 40. 50. heat added (kJ/mol) Use this graph to answer the following questions: What is the boiling point of X ? °C (check all that apply) What phase (physical state) of X would you expect to find in the flask after 12 kJ/mol of heat has been added? solid O liquid gasarrow_forward= STATES OF MATTER Identifying phase transitions on a heating curve A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: temperature (°C) 170. 150. 130. 110. 90 70. 0. 10. What is the melting point of X ? Use this graph to answer the following questions: heat added (kJ/mol) What phase (physical state) of X would you expect to find in the flask after 5 kJ/mol of heat has been added? *C 30. X 40. (check all that apply) solid liquid gas 0/3 50.arrow_forward
- need help with this chemistryarrow_forwardA shiny black solid has a melting point of 114 C. It does not conduct electricity and it does not dissolve in water to form a conducting solution. The black solid is not as hard as an ionic crystal. What type of solid is this substance? Justify your answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY