
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
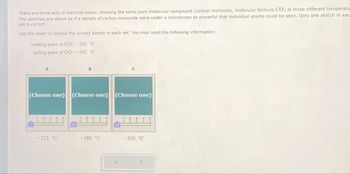
Transcribed Image Text:There are three sets of sketches below, showing the same pure molecular compound (carbon monoxide, molecular formula CO) at three different temperatu
The sketches are drawn as if a sample of carbon monoxide were under a microscope so powerful that individual atoms could be seen. Only one sketch in eac
set is correct.
Use the slider to choose the correct sketch in each set. You may need the following information:
melting point of CO: -205. °C
boiling point of CO:-192. °C
A
C
(Choose one) (Choose one) (Choose one)
3
4
5
-212. °C
-180. °C
-200. °C
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Research studies have found that the price elasticity of demand for bread is equal to -0.40 and the price elasticity of demand of butter is equal to -1.13. Holding everything else constant, if the price of bread and the price of butter each increase by 10 percent: (a) Will the revenue from sales of bread increase or decrease? (b) Will the revenue from sales of butter increase or decrease?arrow_forwardUse and modify some/all the reactions provided to form the indicated overall reaction according to Hess' Law. • If a reaction is not used in your Hess' Law process, include that indication by multiplying the reaction by "0" • If no modification is needed and the reaction is applied as written below, include that indication by multiplying the reaction by "1" • Use whole numbers and simple fractions (such as "2" or "1/2", not 0.5) and negative signs (such as "-2") where needed Then calculate and report the enthalpy change (in kJ) for the overall reaction in the indicated blank, including units with your written value and rounded to 1 decimal place. Overall Reaction: CS2(1) + 302(g) → CO2(g) + 2SO2(g) Rxn # Reaction ΔΗ° (kJ) 1 C(s) → C(g) 716.67 2 C(s) + O2(g) → CO2(g) -393.5 3 3Fe(s) +202(g) → Fe3O4(s) -1118 4 C(s) + 2S(s) → CS2(1) 87.9 5 CH4(g) + O2(g) → CO₂(g) + H₂O(g) -882.0 6 S(s) + O2(g) → SO2(g) -296.8 N2(g) + 4H2(g) + Cl2(g) → 2NH4Cl(g) -629.1 7arrow_forwardWith the information provided answer Carrow_forward
- Is there a reason those sig figs are wrong or are my calculations just completely off?arrow_forwardCalculate (triangle)G for: C2H4 (g) + H2O (g) -> C2H5OH(g)arrow_forwardWhich of the following gases consist of molecules containing four or more atoms? Rn O NO O CO Ar Kr CO2 Хе H,S HF H, F, N, sO, Ne HCN Cl, NO, CH, Не Sa HCI MacBook Proarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY