
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
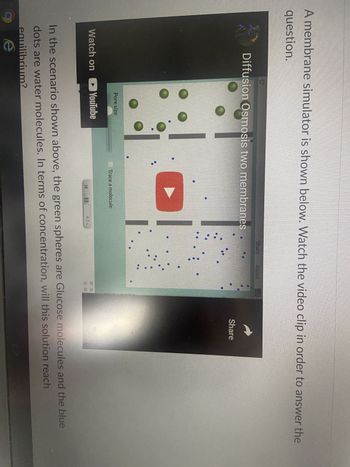
Transcribed Image Text:**Educational Transcript: Membrane Diffusion and Osmosis**
**Section Overview:**
In this section, we explore the concept of diffusion and osmosis across two membranes using a simulation.
**Visual Aid Description:**
- A screenshot from a membrane simulator video demonstrating diffusion and osmosis.
- The screen shows a division with two different areas separated by membranes.
- Green spheres represent glucose molecules, and blue dots represent water molecules.
**Instructions:**
Watch the video clip to understand how molecules move across the membranes and answer the following question:
"In the scenario shown above, the green spheres are glucose molecules, and the blue dots are water molecules. In terms of concentration, will this solution reach equilibrium?"
**Video Controls:**
- Play/Pause button for the video is available.
- A slider controls the "Pore Size" in the simulation, influencing the passage of molecules.
- The video includes tracing options to monitor individual molecules.
Understanding these concepts is crucial for comprehending how substances move in biological systems.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Answer the following questions based on the graph: a) Which of the two molecules that are the same size will passively diffuse across a cell membrane quicker? Why? [2 A] b) Which type of transport method would glucose use? Why? [2 A] Figure 1: Relative Sizes of Molecules Diffusing Across a Cell Membrane Substance Tested water oxygen glycerol glucose alcohol carbon dioxide Graph 50 100 Relative Size of Molecules 150 200arrow_forwardWhat is the role of lysozyme and a low ionic strength extraction buffer? Select all that apply. Cells absorb water from a low ionic strength extraction buffer, by osmosis, swell and lyse, releasing their contents Lysozyme breaks down bonds in the cellulose cell wall of plants, weakening the structure Cells release water into a low ionic strength extraction buffer by osmosis, shrink and lyse, releasing their contents Lysozyme is an enzyme found in lysosomes in cells Lysozyme breaks down bonds in the peptidoglycan cell wall of bacteria, weakening the structure Lysozyme is an antibiotic found in tears, saliva, human milk and mucus.arrow_forwardPassive transport: What are they and examples for each 1. Mechanical 2. Voltage 3. Ligand:arrow_forward
- Structure B is a Extracellular fluid B Nh Cytoplasm transport protein solute phospholipis water molecule low solvent [arrow_forwardDescribe TWO (2) methods transport of solutes in the systems with the aid of diagram I want a clear solution for me to understandarrow_forwardHow do I solve this problem? I tried everything, I need the answer.arrow_forward
- ell Transport What is shown in the picture below? Plasma membrane C Extracellular fluid -Sodium Na Cytoplasm 11,819 10000 www 0 ATP Phosphate ADP #13 Potassium K* mo 00 20 N Na K" Concentrationarrow_forwardPlease see the picture belowarrow_forwardThe plasma membrane has a hydrophobic interior due to the two present in each phospholipid found in the phospholipid bilayer of the cell membrane. The plasma membrane allows some molecules to cross but not all. Therefore, the plasma membrane is said to be Molecules that are nonpolar and hydrophobic will cross the membrane with ease by Does this process require energy? When molecules move across the plasma membrane by passive transport, they will move down or with the_ from an area of concentration to an area of. concentration. Polar, hydrophilic molecules and charged ions will move across the plasma membrane by the process of_ This does not require energy, but it does require a A special case of diffusion is known as which is the movement of water across the plasma membrane. If a cell is placed into a solution that is hypertonic compared to the inside of the cell, the cell would If a cell is placed into a hypotonic solution compared to the inside of the cell, the cell water. would…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON