
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
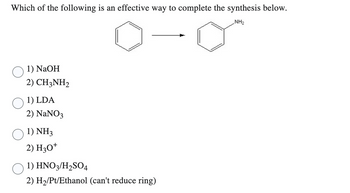
Transcribed Image Text:Which of the following is an effective way to complete the synthesis below.
NH₂
1) NaOH
2) CH3NH2
1) LDA
2) NaNO3
1) NH3
2) H3O+
1) HNO3/H₂SO4
2) H₂/Pt/Ethanol (can't reduce ring)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the mechanism to get from the reactant to the productsarrow_forwardWhich of the following is an effective way to complete the synthes below. - © LĨNH, 2) H₂O* 1) HNO₂H₂50 2) H₂/Ethanol (can't reduce ring) O DILDA 2) NANO 1) NaOH 2)CHÍNH)arrow_forwardCH3−CH2−CH2−CH2−CH2−O||C−NH2+HCl→______+NH4Cl What is the unidentified product of the reaction? Select the correct answer below: CH3−CH2−CH2−CH2−CH2−O||C−OH CH3−CH2−O||C−OH CH3−CH2−CH2−O||C−OH CH3−CH2−CH2−CH2−CH2−CH2−OHarrow_forward
- draw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions.arrow_forwardWhich of the following compounds reacts faster in an elimination reaction with KOH? Also provide the major product(s) (if any) for each elimination reaction. Br Br )...arrow_forwardfrom compound 3 to 4, the nitro group in compound 3 is going through [ hydrolysis / reduction / substitution / oxidation] to afford compound 4arrow_forward
- please answer in text form and in proper format answer with must explanation , calculation for each part and steps clearlyarrow_forwardHello, If you could please help me solve this problem. These are the options for each reagent. 1: HCl/H2OHCl/H2O CH3NH2CH3NH2 NaOH/H2ONaOH/H2O methanol 2: NaOH/H2O CH3NH2 methanol HCl/H2O 5. NaOH/H2O CH3NH2 methanol HCl/H2O 6 NaOH/H2O CH3NH2 methanol HCl/H2Oarrow_forwardPlease don't provide handwritten solutionarrow_forward
- Draw the MAJOR organic product(s) for each of the following reactions/sequence of reactions. OEt ΕΙΟ OEt OH 1) PCl3 / Br₂ NH2 2) / pyridine 1) EtONa / EtOH 2) H3O+ (dilute) 1) EtONa / EtOH 2) Ph 3) NaOH / H₂O Br 4) H2SO4/H₂O/heat OH 1) SOCI₂ 2) HO- / Et₂N NO2 1) MCPBA / CF3SO₂H 2) Ph-NH2/pyridine 1arrow_forwardProvide the major organic product in the reaction below. H OH H POCI3 pyridinearrow_forwardCan you please tell me the reagents/conditions of each step 1, 2, 3 and possible occuring problems (for example: side reaction)? If there's any issues, please explain why the step works well.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY