
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
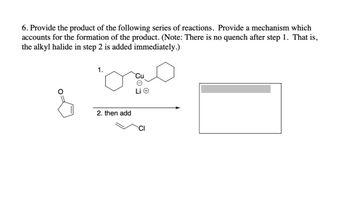
Transcribed Image Text:6. Provide the product of the following series of reactions. Provide a mechanism which
accounts for the formation of the product. (Note: There is no quench after step 1. That is,
the alkyl halide in step 2 is added immediately.)
1.
میں
&
2. then add
CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Consider the Friedel-Crafts (FC) alkylation of N-phenylacetamide (shown below) in which a reaction with 1-chloropropane and AlCl3 produces two isomers of isopropyl N-phenylacetamide. 1. Draw the complete reaction mechanism for the formation of the ortho product (para is also formed). 2. Is this reaction faster or slower than the similar FC reaction starting with benzene (C6H6)? Explain why. 3. Explain why this reaction produces the ortho and para products (but not meta). Use your mechanism to help explain parts #2 and #3. Use additional drawings and words as needed.arrow_forwardThis is a umplong reaction. I'm looking for what reagent and intermediates would look like. Thank youarrow_forwardGive the major product of the following reactions. This is one question just consists of multiple parts.arrow_forward
- 5. Synthesis a) Show how to make the following ether starting from Propylchloride and 2-lodopropane, keep in mind that secondary alkyl halides eliminate with nucleophiles that are strong bases to give you the alkene products. CIarrow_forward1. Provide the mechanism for the following reaction. OH H2SO4 HeAt CH2 CH3 CH3arrow_forwardShow an example of this mechanism using this general reaction.arrow_forward
- 4. Propose an efficient synthetic route (along with intermediates) for the following transformation. Note: Use cyclohexane as the starting point for both cyclohexyl rings in the product.arrow_forwardQ4. Alkyl halides are good at doing substitution (SN2/SN1) and elimination (E1/E2) reactions because the halide (X-) is a good LG. Alcohols on the other hand are not suitable for these reactions since hydroxide (¯OH) is a poor LG. However, an OH can be converted into a OTs (tosyl group) by reacting an alcohol with TsCl in presence of a base. An OTs is a good LG, and this allows us to indirectly (via OTs) use alcohols in substitution (SN2/SN1) and elimination (E1/E2) reactions. (4) OH TsCl NEt3 OTS Tosyl ester NaCN DMSO + HNEt3 Determine the major/minor products CH3OH heat OTS Tosyl ester TsCl = NaOCH3 CH3OH DBU Ś=0 CIarrow_forward5. Provide the product that will result for the following reaction and provide the complete mechanism. CH,N2 НО 오arrow_forward
- Rank the following compounds in order of increasing acidityarrow_forward3. Perform the following tasks for each reaction. i. Identify the functional group (FG) in the starting material with the most accurate name: alcohol, aldehyde, epoxide, ester, ether, or halohydrin. ii. Label the reagent over the arrow as a good nucleophile; a strong base; and/or a strong acid. (A reagent can have more than one label.) Draw the major organic product. (Hint: Remember to keep track of stereochemistry over the course of the reaction if given. It may be helpful to redraw the starting material in the conformation needed to close epoxide ring.) ii. iii. Product i. Starting Material OH ii. Reagent a. D. NaOH FG = b. Excess HBr FG = с. 1. Li(CH2);CH3 2. H3O* (Acidic workup for neutralizing charge) FG = d. ОН NaH FG = е. HBr FG =arrow_forwardDon’t understand 7arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY