
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
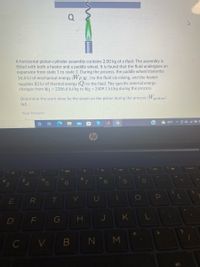
Transcribed Image Text:Q
A horizontal piston-cylinder assembly contains 2.00 kg of a fluid. The assembly is
fitted with both a heater and a paddle wheel. It is found that the fluid undergoes an
expansion from state 1 to state 2. During the process, the paddle wheel transmits
16.4 kJ of mechanical energy (Wp.w.) to the fluid via mixing, and the heater
supplies 83 kJ of thermal energy (Q) to the fluid. The specific internal energy
changes from U1 = 2386.6 kJ/kg to U2 = 2409.1 kJ/kg during the process.
Determine the work done by the steam on the piston during the process (Wpiston)
(kJ).
Your Answer:
45°F
hp
Insert
*
24
%
8.
4
R
H
J
K
D
F
CV
B N
M
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Help me with this thermodynamics problemarrow_forwardPatm 1. Initially; the piston sits on the stops and the linear spring exerts no force, the specific volume is 0.05 m /kg and the pressure is 125 kPa. Atmospheric pressure is 100 kPa, mass of the piston is 102 kg, and area of the piston is 200 cm?. The linear spring exerts a force on the piston in such a way that the specific volume becomes 0.08 m'/kg when the pressure reaches 200 kPa. Heat is transferred to the water until its temperature is 115 °C. Calculate work and heat transfer, showing the process on a P-v plot. Hint: In the final stage, the temperature is 115°C, but the pressure is not given. You need to find P and v using the tables and P-v plot.arrow_forwardPlease help me with number 10. If mass needs 600,000 ft-lb/lb of work at atmospheric pressure to evaporate until it occupy a volume of 250 ft^3. Find the mass m.arrow_forward
- T10a please help me with the answer and full solutionarrow_forwardB/ Water in a rigid, insulating tank is set in rotation and left. Water comes to rest after some time due to viscous forces. Considering the tank and water to constitute the system answer yes or no. (i) Is any work done during the process of water coming to rest? (ii) Is there a flow of heat? (iii) Is there any change in internal energy (U)? (iv) Is there any change in total energy (E)?arrow_forwardIn the two-pipe system, water flows continuously from the inner pipe and air from the outer pipe (steady-state). ˙The pressure change in both pipes is negligible. In addition, heat interaction with the environment, kinetic and potential energy changes are also neglected. In this system shown in Figure 1, the water flowing from the inlet number 1 is in the state of saturated vapor (gas). In this case, calculate the outlet temperature (T2) for the given system, using the ideal gas approximation for air. (Hint: it will be solved iteratively.)arrow_forward
- In the two-pipe system, water flows continuously from the inner pipe and air from the outer pipe (steady-state). ˙The pressure change in both pipes is negligible. In addition, heat interaction with the environment, kinetic and potential energy changes are also neglected. In this system shown in Figure 1, the water flowing from the inlet number 1 is in the state of saturated vapor (gas). In this case, calculate the outlet temperature (T2) for the given system, using the ideal gas approximation for air. (it should be solved iteratively.) (Please solve it on paper and solve it line by line. When you type it, it becomes too complicated to understand. Also please explain your solution and don't write too close. Thank you for your effort)arrow_forwardA water tube boiler has a capacity of 1000 kg/hr of steam. The factor of evaporation is 1.3, boiler rating is 200%, boiler efficiency is 5%, heating surface area is 0.91 m²/boiler Hp, and the heating value of fuel is 18,400 Kcal/kg. The total coal available in the bunker is 50,000 kg. Determine total number of hours to consume the available fuel. 1.arrow_forwardTHE PRESSURE IN THE SUBTANCE DRASTICALLY DECREASE FROM 354 TO 21 POUNDS PER SUARE INCHES (PSI), THE HEAT ENERGY OF THE OPEN SYSTEM REDUCES 555BTU/LB, AND THE VOLUME INCREASES FROM 1 TO 10FT^3/LB. (A) DETERMINE THE WORK PER LB. UNITS:BTU/LB * (B) DETERMINE THE WORK IN HP (HORSE POWER) FOR 10LB PER MIN. (1HP = 42.4BTU/MIN). UNITS:HParrow_forward
- Please answer correct and neatarrow_forwardQ3. The data listed in the following table gives hourly measurements of heat flux q (cal/cm? /h) at the surface of a solar collector. As an architectural engineer, you must estimate the total heat absorbed by a 150,000-cm2 collector panel during a 14-h period. The panel has an absorption efficiency eab of 45%. The total heat absorbed is given by: h = eab q A where A = area and g = heat flux. 4 6. 10 12 14 0.10 5.32 7.80 8.00 8.03 6.27 3.54 0.20arrow_forwardsolve in 15 mins i will give thumb uparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY