
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
If the cylinder is opened and the gas allowed to escape into a large empty plastic bag, what will be the final volume of nitrogen gas, including what's collected in the plastic bag and what's left over in the cylinder? Write your answer in liters. Be sure your answer has the correct number of significant digits.
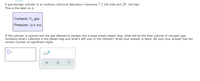
Transcribed Image Text:A gas storage cylinder in an ordinary chemical laboratory measures 7.2 cm wide and 29. cm high.
This is the label on it.
Contents: N, gas
Pressure: 18.6 atm
If the cylinder is opened and the gas allowed to escape into a large empty plastic bag, what will be the final volume of nitrogen gas,
including what's collected in the plastic bag and what's left over in the cylinder? Write your answer in liters. Be sure your answer has the
correct number of significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemical engineer must report the average volume of a certain pollutant produced by the plants under her supervision. Here are the data she has been given by each plant: plant Allegheny Platte Doheny L volume of pollutant 40.1 L 0.369 L 2.947 L What average volume should the chemical engineer report? Be sure your answer has the correct number of significant digits. ☐x10arrow_forward511 mL of oxygen gas at a temperature of 113 degrees Celsius contracts to 450 mL upon cooling. What is the new temperature in degrees Celsius? Use 273 for the conversion of Celsius to Kelvin. Do not type units in your answer. If the final answer is negative be sure to include the negative sign. Your Answer:arrow_forwardThe temperature of a 9.48 ×102 g air sample (density = 1.19 g/L) was lowered, and the density increased to 1.92 g/L. Calculate the new volume of the air sample. = how many literarrow_forward
- A chemical engineer must report the average volume of a certain pollutant produced by the plants under her supervision. Here are the data she has been given by each plant: plant Lincoln New Bedford Macon OL volume of pollutant 0.43 L 33.46 L 35.18 L What average volume should the chemical engineer report? Be sure your answer has the correct number of significant digits. x10 X Śarrow_forwardAn arctic weather balloon is filled with 21.7 L of helium gas inside a prep shed. The temperature inside the shed is 9. °C. The balloon is then taken outside, where the temperature is -32. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Round your answer to 3 significant digits. L ☐ X 3arrow_forwardGaseous ethane CH3CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. Suppose 19. g of ethane is mixed with 52.1 g of oxygen. Calculate the minimum mass of ethane that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forward
- An arctic weather balloon is filled with 26.0 L of helium gas inside a prep shed. The temperature inside the shed is 15. °C. The balloon is then taken outside, where the temperature is -12. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits. 0 x10 X Śarrow_forwardGaseous methane (CH4) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 0.64 g of methane is mixed with 3.84 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. 8.0 0 x10 X S MacBook Air Submit © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardAn arctic weather balloon is filled with 12.0 L of helium gas inside a prep shed. The temperature inside the shed is 15. °C. The balloon is then taken outside, where the temperature is - 16. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits. L 0 x10 X Śarrow_forward
- Gaseous methane CH4 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. Suppose 7.1 g of methane is mixed with 16.3 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forwardAqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium chloride NaCl and liquid water H2O . Suppose 28. g of hydrochloric acid is mixed with 53.0 g of sodium hydroxide. Calculate the minimum mass of hydrochloric acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY