
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
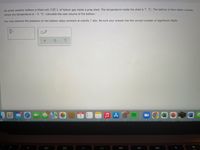
Transcribed Image Text:An arctic weather balloon is filled with 3.02 L of helium gas inside a prep shed. The temperature inside the shed is 7. °C. The balloon is then taken outside,
where the temperature is -5. °C. Calculate the new volume of the balloon.
You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits.
L
ЛА
80
DII
DD
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An arctic weather balloon is filled with 42.3 L of helium gas inside a prep shed. The temperature inside the shed is 11. °C. The balloon is then taken outside, where the temperature is – 12. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits. olo x10 ? 18 Ararrow_forwardGaseous methane CH4 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . Suppose 1.44 g of methane is mixed with 3.1 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. garrow_forwardAn arctic weather balloon is filled with 44.0 L of helium gas inside a prep shed. The temperature inside the shed is 10. °C. The balloon is then taken outside, where the temperature is -24. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits. x10 ?arrow_forward
- An arctic weather balloon is filled with 15.7 L of helium gas inside a prep shed. The temperature inside the shed is 15. °C. The balloon is then taken outside, where the temperature is -41. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits. Ox10arrow_forwardCalculate the new volume of the balloon. Round your answer to 3 significant digits.arrow_forwardGaseous methane (CH,) will react with gaseous oxygen (O,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 14. g of methane is mixed with 77.9 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. x10arrow_forward
- Liquid hexane CH3CH24CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. Suppose 19. g of hexane is mixed with 38.1 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forwardAn arctic weather balloon is filled with 6.80 L of helium gas inside a prep shed. The temperature inside the shed is 12. °C. The balloon is then taken outside, where the temperature is -16. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Round your answer to 3 significant digits. 0 X S ? olo Ararrow_forwardA chemical engineer must report the average volume of a certain pollutant produced by the plants under her supervisio Here are the data she has been given by each plant: volume of plant pollutant Lincoln 0.521 L Doheny 3.453 L Allegheny 1.654 L What average volume should the chemical engineer report? Be sure your answer has the correct number of significant digits. x10 ?arrow_forward
- A chemical engineer must report the average volume of a certain pollutant produced by the plants under her supervision. Here are the data she has been given by each plant: plant Allegheny Platte Doheny L volume of pollutant 40.1 L 0.369 L 2.947 L What average volume should the chemical engineer report? Be sure your answer has the correct number of significant digits. ☐x10arrow_forwardThe temperature of a 9.48 ×102 g air sample (density = 1.19 g/L) was lowered, and the density increased to 1.92 g/L. Calculate the new volume of the air sample. = how many literarrow_forward(CONTINUED ON NEXT PAGE) 4. Let's say that the full volume of your flask is 325 ml. You heat the flask of air in a hot water bath at 89.0°C according to your thermometer. You then inverti into the cold water bath at 5.0°C Water enters the flask as the volume of the gas shrinks. a) Theoretically, what should the volume of the gas be after it sits in the cold water bath? b) You find that during your experiment that 65.0 mL of water has entered the flask. What is the experimental volume of the gas? c) What is your percent error for this measurement?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY