
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
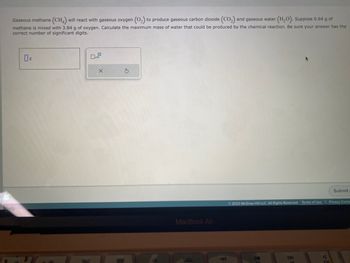
Transcribed Image Text:Gaseous methane (CH4) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 0.64 g of
methane is mixed with 3.84 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the
correct number of significant digits.
8.0
0
x10
X
S
MacBook Air
Submit
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- During an accident, the air bag inflates due to the decomposition reaction of sodium azide (NaN3). When heated, solid sodium azide reacts by itself to form solid sodium and nitrogen gas as products. What mass of sodium azide do you need to produce 200. L of nitrogen gas. The density of nitrogen gas is 1.2056 g/L.arrow_forwardGaseous butane (CH,(CH,) CH;) will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (Co,) and gaseous water (H,O). Suppose 3.49 g of butane is mixed with 23. g of oxygen. Calculate the minimum mass of butane that could be left over by the chemical reaction. Round your answer to 2 significant digits. Ox10 ?arrow_forwardA mixture of barium chloride and sulfuric acid yield the precipitate barium sulfate and hydrochloric acid according to the double replacement reaction: A large quantity of barium chloride is used to ensure that the sulfuric acid is the limiting reactant. If 45.0 grams of barium sulfate are produced, what mass of H2SO4 must be dissolved in the initial aqueous solution? _ g [Number in standard notation; round your answer to one decimal place] The following molar masses may or may not be needed, but are provided to save you time: barium sulfate = 233 g/mol sulfuric acid = 98.0 g/molarrow_forward
- Liquid octane (CH₂(CH₂) (CH3) reacts with gaseous oxygen gas to produce gaseous carbon dioxide (CO₂) and 6 gaseous water (H₂O). If 23.2 g of water is produced from the reaction of 107.36 g of octane and 150.39 g of oxygen gas, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.arrow_forwardB. Part C Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2A1(s)+3C12 (g)→2AIC13 (s) What is the maximum mass of aluminum chloride that can be formed when reacting 28.0 g of aluminum with 33.0 g of chlorine? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) unt of O圖] ? Value Units Submit Provide Feedback Next > 9. |国6 MacBook Pro G Search or type URL % * 5. delet 7. 8. R. P. 1 K. 21 option KUzy commandarrow_forwardPropane is a hydrocarbon that fuels everything from gas grills to home furnaces. Below is the balanced chemical equation for the combustion of propane (C3H8): C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2O If you have 410 grams of propane and want to know how many grams of oxygen are required to burn it, you can follow these steps… Find the number of moles of propane that you have, then convert grams to moles! (1 mol ÷ 44 g )×410 g = 9.32 mol of 5×.9.32 mol oxygen = 46.6 mol of oxygen The moles of propane are related to the moles of oxygen by the molar ratio (ratio of coefficients in the balanced chemical equation). Find the number of moles of oxygen you need to give the moles of propane from part 5a. 1 mol oxygen molecule = 32 g of oxygen 46.6 mol of oxygen = 32 g × 46.6 mol of oxygen = 1491.2 g of oxygen Find the grams of oxygen from the moles of oxygen. Convert the moles of oxygen (answer to part 5b) to grams of oxygen (O2)! Hint: use the molar mass for O2, not just O. You…arrow_forward
- Hand written solutions are strictly prohibitedarrow_forwardAqueous hydrobromic acid HBr reacts with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O . If 1.82g of water is produced from the reaction of 15.4g of hydrobromic acid and 10.0g of sodium hydroxide, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.arrow_forwardChlorine gas (C1₂) reacts with solid calcium (Ca) to produce solid calcium chloride (CaCl₂). Write a balanced chemical equation for this reaction. 0 0-0 X 2 5 Sarrow_forward
- 3.7 x 1021 water molecules has a mass of _____ grams. Keep four decimal places A drop of water has a mass of 0.043 grams. Find the number of water molecules in this sample (N). Calculate log10N. Example: log10(3.5 x 1021) = 21.54arrow_forwardAqueous hydrobromic acid HBr reacts with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O . If 0.321g of water is produced from the reaction of 3.2g of hydrobromic acid and 2.1g of sodium hydroxide, calculate the percent yield of water.Be sure your answer has the correct number of significant digits in it.arrow_forwardLiquid hexane CH3CH24CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . What is the theoretical yield of water formed from the reaction of 79.3g of hexane and 213. g of oxygen gas? Be sure your answer has the correct number of significant digits in it.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY