
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
pls check my answers
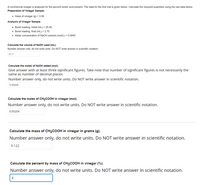
Transcribed Image Text:A commercial vinegar is analyzed for the percent acetic acid present. The data for the first trial is given below. Calculate the required quantities using the raw data below.
Preparation of Vinegar Sample:
• Mass of vinegar (g) = 3.06
Analysis of Vinegar Sample:
• Buret reading, initial (mL.) = 25.40
• Buret reading, final (mL) = 3.70
• Molar concentration of NAOH solution (mol/L) = 0.0940
Calculate the volume of N2OH used (mL).
Number answer only, do not write units. Do NOT write answer in scientific notation.
21.7
Calculate the moles of N2OH added (mol).
Give answer with at least three significant figures. Take note that number of significant figures is not necessarily the
same as number of decimal places.
Number answer only, do not write units. Do NOT write answer in scientific notation.
0.00204
Calculate the moles of CH3COOH in vinegar (mol).
Number answer only, do not write units. Do NOT write answer in scientific notation.
0.00204
Calculate the mass of CH3COOH in vinegar in grams (g).
Number answer only, do not write units. Do NOT write answer in scientific notation.
0.122
Calculate the percent by mass of CH3COOH in vinegar (%).
Number answer only, do not write units. Do NOT write answer in scientific notation.
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- urgent pleasearrow_forwardOII mL Correct: 0 Wrong: 0 Zoomed View Check 1000 900 800 7 8 9. 800 700 700 4 6. 600 500 600 400 1 3 300 500 200 100 400arrow_forwardQuestion 1 of 20 A barrel of crude oil has a volume of 42 gallons, only approximately 45% of which is processed into gasoline. If your car achieves 32 mi/gal, and you drive 36,000 miles in one year, how many barrels of crude oil are required to run your car for a year? Tap here or pull up for additional resourcesarrow_forward
- Search he web.. H Take T X G What is th P Pearson S b Answered M Inbox (1,0 er 1 0 8 https://bblearn.rcsj.edu/webapps/assessment/ Question Completion Status: 3. 50 70 100 110 120 130 14G 18 QUESTION 3 3.478 g x 1.164 g What is the best answer to report for -0.116 g/mL g/mL? 2.00 mL O a. 1 g/mL O b.1.904 g/mL Oc.1.90478009 g/mL O d. 1.9 g/mL Oe1.91 g/mL QUESTION 4 The state of matter for an object that has both definite volume and definite shape i a. gaseous state b. elemental state OC. solid state Od. mixed state e. liquid state Click Save and Submit to scue and subrt CackSae74nswers to save al cnswers. Type here to searcharrow_forwardPart D a solution with a density of 1.01 g/mL that is 1.11% HCl by mass Express your answer to three decimal places. 4 5 ΑΣΦ pH = Submit Provide Feedback Q Search Request Answer hparrow_forwardQUESTION 7 Find the incorrect equality O 1h = 3600 s O 1 gal = 4 qt %3D O 5 mL = 1 tsp %3D O 1L= 1000 mm QUESTION 8 What is the conversion factor for thearrow_forward
- Mr. Z measured the pH of the pond water in USC pond. His measurements are as follows: 8.1, 7.8, 8.2, 7.9, 8.0, 8.3 If the true pH of the pond water is 8.2, a) Calculate the absolute error. b) Calculate the relative error c) Calculate the percent relative error d) Calculate the standard deviation e) Calculate the relative standard deviationarrow_forwardQuestion 3 The literature value of R is 0.08206 Latm/molK. Your experimental value is 0.08012. What is your percent error? 920% 2.36% 92.0% 0236% 70°Farrow_forwardQuestion A graduated cylinder initially contains 48.6 mL water. When a piece of jewelry is submerged in the graduated cylinder, the total volume increases to 61.2 mL. What is the volume of the piece of jewelry? Select the correct answer below: O109.8 cm³ O61.2 mL O 12.6 cm³ 48.6 cm³ Content attribution O C H U 8 411 FEEDBACK MORE INSTRUCTION + 8 SUBMIT Rain to stop ←backspace 7arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY