
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Search he web..
H Take T X
G What is th
P Pearson S
b Answered
M Inbox (1,0
er 1
0 8 https://bblearn.rcsj.edu/webapps/assessment/
Question Completion Status:
3.
50
70
100 110
120
130
14G
18
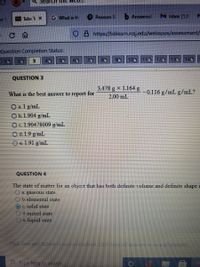
QUESTION 3
3.478 g x 1.164 g
What is the best answer to report for
-0.116 g/mL g/mL?
2.00 mL
O a. 1 g/mL
O b.1.904 g/mL
Oc.1.90478009 g/mL
O d. 1.9 g/mL
Oe1.91 g/mL
QUESTION 4
The state of matter for an object that has both definite volume and definite shape i
a. gaseous state
b. elemental state
OC. solid state
Od. mixed state
e. liquid state
Click Save and Submit to scue and subrt CackSae74nswers to save al cnswers.
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following prompt will be used to answer the first six questions. An experiment was performed to separate the components of a binary mixture composed of sucrose (sugar) and sand. The following data was collected in lab during the experiment. Mass of original binary mixture: 20.100 ± 0.001 g Mass of initial filter paper: 21.206 ± 0.001 g Mass of filter paper and isolated sand: 25.226 ± 0.001 g q1 Given the data and information in the prompt, what percent of sand is in the original mixture with absolute error? You will submit your answer in three parts. For this question, what value do you get for the percent of sand? You only need to submit the value here since the sig figs and error will be entered in the next two questions. You do not need to include the % symbol with your answer.arrow_forwardThe following prompt will be used to answer the first six questions. An experiment was performed to separate the components of a binary mixture composed of sucrose (sugar) and sand. The following data was collected in lab during the experiment. Mass of original binary mixture: 20.100 ± 0.001 g Mass of initial filter paper: 21.206 ± 0.001 g Mass of filter paper and isolated sand: 25.226 ± 0.001 g q3 Given the data and information in the prompt, what is the calculated absolute error on the percent of sand in the mixture? Enter the absolute error you calculated in the box below. You do not need to include the % symbol with your answer.arrow_forward19. List four properties you could use to distinguish between iron and aluminum. State which of them are chemical properties. 20. List four properties you could use to distinguish between water and gasoline. State which of them are chemical properties.arrow_forward
- Number 7 and please explainarrow_forwardQUESTION 15 Match the correct prefix with the factor. in A. 10-9 k B. 103 C. 102 D. 109 E. 10-2 F. 103arrow_forwardPart A You are given the masses, in grams (g), and the volumes, in cubic centimeters (cm³), for a series of substances. Arrange them in order of increasing density. Rank from lowest density to highest density. To rank items as equivalent, overlap them. View Available Hint(s) NaCl 43 g 20 cmi Lowest density Copper 44.5 g 5 cm The correct ranking cannot be determined. 1921 JUN 30 Gold 19.3 g 1 cm³ Ice 92 8 100 cm Butane 2895 g 5000 cm³ tv NA CHELTEN MacBook Air Reset Chloroform 14.8 g 10 cm² Help Highest density AO W Xarrow_forward
- A nutrition table lists apples with a potassium content of 4600 mg/pound. Assume an average apple is 75.0 g, the minimum daily intake for potassium for a healthy adult is 3500. mg per day. Calculate the number of apples per day to meet this requirement for a healthy adult. Assume 454 g = 1 pound. O 7.96 apples per day O 0.126 apples per day O 0.217 apples per day O 4.61 apples per dayarrow_forwardQuestion 29 of 32 Submit A hollow spherical iron ball has a diameter of 15.3 cm and has a mass of 13.5 kilograms. Assuming the hole inside the ball is spherical with the same center as the center of the ball, what is the thickness in cm of the layer of iron surrounding the hole? The density of iron is 7.86 g/cm³. (The volume of a sphere is (4/3)Trr.) cm 1 2 3 4 6. C 7 8 9. +/- x 100 + LOarrow_forwardAn analytical chemist measures the amount of Elements E, and E, in four samples of an unknown Substance X: sample of E mass mass of E₂ 1 3.5 g 18.5 g 7.6 g 19.4 g 3.4 g 17.6 g 2.2 g 11.8 g Using this information, answer the questions in the table below. 2 3 4 It's known that contains no elements other than E, and E Is a pure substance or a mixture? If you don't have enough information to decide, choose can't decide. If you said X'is a pure substance, calculate the mass of Element E, the analytical chemist would find in a new 10.0 g sample of X. Round your answer to 2 significant digits. Opure substance O mixture O (can't decide) 08 0.8 Xarrow_forward
- Please answer question number 2 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. (please show all work) Question 8: Calculate the mass in grams of each of the following: C.) 0.00100 mol silicon tetrahydride (SiH4) D. ) 1.50 x 10^-2 mol molecular oxygen (O2) E. 2.30 mol ethylene glycol (C2H6O2)arrow_forwardMass of one a mu)Carbon Type 1 12.0Carbon Type 2 13.0 What is the average mass of carbon in this sample? Is this number on the periodic table?( it may be rounded off ) where?arrow_forwardif the density of an element 1.5 g/ml with volume 1.8 L, its mass will be ... g. 2700 O 2.7 1200 1.2 Non of the abovearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY