
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A clown at a birthday party has brought along a helium cylinder, with which he intends to fill balloons. When full, each balloon contains 0.00440 m3 of helium at an absolute pressure of 1.10 x 105 Pa. The cylinder contains helium at an absolute pressure of 1.90 x 107 Pa and has a volume of 0.00320 m3. The temperature of the helium in the tank and in the balloons is the same and remains constant. What is the maximum number of people who will get a balloon?
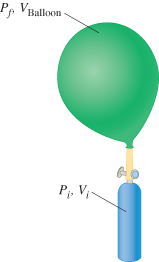
Transcribed Image Text:Pp VBalloon
P₁, V₁
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A container holds 0.410 m3 of oxygen at an absolute pressure of 4.20 atm. A valve is opened, allowing the gas to drive a piston, increasing the volume of the gas until the pressure drops to 1.10 atm. If the temperature remains constant, what new volume (in m3) does the gas occupy?arrow_forwardThe volume of an ideal gas is held constant. Determine the ratio P2/P, of the final pressure to the initial pressure when the temperature of the gas rises (a) from 41 to 82 K and (b) from 21.9 to 54.3 °C. (a) P2/P1 = Number i Units (b) P2/P13D Numberi Unitsarrow_forwardA 0.5 m3 container holds 50 mol of an unknown gas at a temperature of 25 °C. A piston is used to expand the volume of the chamber to 1.0 m3 without changing the temperature of gas. What is the pressure in the container now (in kPa)? The piston is again used to change the volume of the container (without changing the temperature). After doing so the pressure in the container is 496 kPa. What is the volume of the container (in m3)?arrow_forward
- A cylindrical glass beaker of height 1.256m rests on a table. The bottom half of the beaker is filled with a gas, and the top half is filled with liquid mercury that is exposed to the atmosphere. The gas and mercury do not mix because they are separated by a frictionless, movable piston of negligible mass and thickness. The initial temperature is 277 K. The temperature is increased until a value is reached when one-half of the mercury has spilled out. Ignore the thermal expansion of the glass and mercury, and find this temperature in kelvins. Number Unitsarrow_forwardn = 3.9 moles of an ideal gas are pumped into a chamber of volume V = 0.125 m3. Part (a) The initial pressure of the gas is 1 atm. What is the initial temperature (in K) of the gas? Part (b) The pressure of the gas is increased to 10 atm. Now what is the temperature (in K) of the gas?arrow_forwardA gas chamber is to be designed to separate two gases with the following specifications: Hot gas temperature 1145°C Cold gas temperature 45°C Hot gas heat transfer coefficient 230 W/m² K Cold gas heat transfer coefficient 290 W/m² K Metal wall thermal conductivity 115 W/m K If the maximum temperature of the wall on the hot side does not exceed 545°C, using thermal resistance concept, what thickness should the metal wall between the hot gas and cold gas be in mm?arrow_forward
- You are inflating the tires on your bicycle using a manual pump. The volume inside of the pump is initially 0.16 m cube with a pressure of 2 atm. If you push down on the piston and reduce the volume inside to 0.07 m cube, what is the new pressure inside of the pump? Assume the temperature inside the pump stays constant.arrow_forwardThe number density in a container of neon gas is 4.80×1025 m^−3. The atoms are moving with an rms speed of 680 m/s . What is the pressure inside the container? p= 2.46×105 Pa What is the temperature inside the container?arrow_forwardThe gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), and volume V (in liters) is PV = nRT, where n is the number of moles of the gas and R = 0.0821 is the gas constant. Suppose that, at a certain instant, P = 9.0 atm and is increasing at a rate of 0.15 atm/min and V = 13 L and is decreasing at a rate of 0.17 L/min. Find the rate of change of T with respect to time at that instant if n = 10 mol. (Round your answer to four decimal places.) K/min dT_ dtarrow_forward
- Two containers of equal volume each hold samples of the same ideal gas. Container A has twice as many molecules as container B. If the gas pressure is the same in the two containers, the correct statement regarding the absolute temperatures TA and TB in containers A and B, respectively, is TA = TB TA = 2TB %3D TA = TB/2 TA= 1B//2 TA= TB/4 %3Darrow_forwardOxygen with mass of m1=6 g and temperature T1=300 K is in a container under pressure p1= 9.66·105 Pa. If an unknown gas with mass m2= 3 g and temperature T2=330 K is placed in the same container, then the gas is under pressure p2=9·105 Pa. Find the molar mass of the unknown gas.arrow_forwardThe volume of an ideal gas is held constant. Determine the ratio P₂/P₁ of the final pressure to the initial pressure when the temperature of the gas rises (a) from 54 to 108 K and (b) from 35.0 to 66.5 °C. (a) P₂/P₁ = Number i (b) P2/P₁ = Number i Units Unitsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON