Question
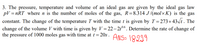
Transcribed Image Text:3. The pressure, temperature and volume of an ideal gas are given by the ideal
pV = nRT where n is the number of moles of the gas, R=8.314 J/(mol× K) is the gas
gas
law
constant. The change of the temperature T with the time t is given by T = 273+43/t . The
change of the volume V with time is given by V = 22– 2t0°. Determine the rate of change of
of 1000 moles gas with time at t = 20s . ANS= 18999
0.6
the
pressure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- The rms speed of the molecules in 1.2 g of hydrogen gas is 1800 m/s. Part A What is the total translational kinetic energy of the gas molecules? Express your answer with the appropriate units. Etotal = 1.9 kJ Submit ✓ Correct Part B Previous Answers What is the thermal energy of the gas? Express your answer with the appropriate units. Eth = 1944 Submit μA Previous Answers Request Answerarrow_forwardQuestion A2 An ideal gas occupies a volume of 1.75 cm³ at 30°C and atmospheric pressure. Calculate the number of molecules of gas in the container. (1 atm = 1.013 x 105 Pa)arrow_forward0.52 mol of argon gas is admitted to an evacuated 3.00 liter (3.00 × 10-3 m3) container at 20.0°C. The gas then undergoes an isobaric process to a temperature of 260°C. What is the final volume of the gas, in liters? Your answer needs to have 3 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement.arrow_forward
- Two containers of equal volume each hold samples of the same ideal gas. Container A has 2 times as many molecules as container B. If the gas pressure is the same in the two containers, find the ratio of the the absolute temperatures TA and TB ( i.e TA / TB ) . Calculate to 2 decimals.arrow_forwardIf gas pressure (absolute value) for an ideal gas is held constant, the relationship between gas temperature and volume is direct inverse of the form y = mx + c O parabolicarrow_forward1. Three moles of a monoatomic ideal gas are placed in a portable container with a volume of 4.10 x 10¬³ m3. The temperature of the gas is 165 °C. What is the average kinetic energy per molecule? sf60 s 60 ssfoc sf60 st ssf60arrow_forward
- A balloon is filled with helium gas at atmospheric pressure (1 atm) until its volume is 800 m³. The helium gas is then transferred to cylinders that have a volume of 2.3 m³ at a pres- sure of 13.3 atm. Calculate the number of cylinders used. Assume that the temperature of the helium gas remains constant. (1 atm = 1.013 × 105 Pa).arrow_forwardA hot air balloon uses the principle of buoyancy to create lift. By making the air inside the balloon less dense then the surrounding air, the balloon is able to lift objects many times its own weight. A large hot air balloon has a maximum balloon volume of 2090 m3. a. If the air temperature in the balloon is 54 °C, how much additional mass, in kilograms, can the balloon lift? Assume the molar mass of air is 28.97 g/mol, the air density is 1.20 kg/m3, and the air pressure is 1 atm.arrow_forwardA fixed amount of a particular ideal gas at 20°C and a pressure of 1.75 × 105 Pa occupies a volume of 2.60 m3. If the volume is increased to 4.2 m³ and the temperature is raised to 27.0°C, what will be the new pressure (in Pa) of the gas? Paarrow_forward
arrow_back_ios
arrow_forward_ios