
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
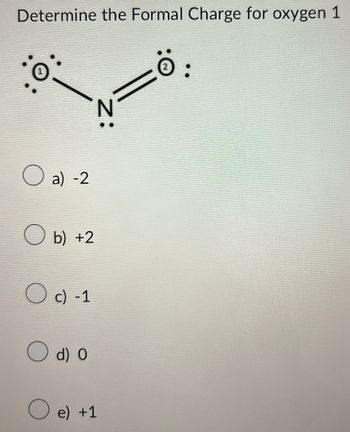
Transcribed Image Text:Determine the Formal Charge for oxygen 1
N:
a) -2
b) +2
c) -1
d) O
e) +1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Draw the Lewis structure of NCO (with minimized formal charges) and then choose the appropriate formal charges for each of the atoms. A) N = 0, C = 0, O = -1 B) N = -1, C = +1, O -1 + C) N = 0, C = 0, O = 0 D) N = +1, C = -1, O = -1 E) N = -1, C = 0, O = 0arrow_forwardIn the Lewis structure for cyanate ion given below, what is the formal charge on the carbon atom?arrow_forwardIodine forms a series of fluorides (listed here). Write Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule:(a) IF(b) IF3(c) IF5(d) IF7arrow_forward
- Draw all possible resonance structures for each of these compounds. Determine the formal charge on each atom in each of the resonance structures:(a) O3(b) SO2(c) NO2 −(d) NO3−arrow_forwardCalculate the formal charge of the following molecules. H₂C H₂O+ NH H/₂C=N+arrow_forwardGiven two possible resonance forms of NCS and the associated formal charges. Predict whether a or b is more stable. (a) (b) :N=C=S: c=N=s: -1 0 0 -2 +1 0arrow_forward
- Draw a Lewis diagram for BrO4- in which the central Br atom has a formal charge of zero and show all NONZERO formal charges on all atoms. Note that the overall charge on this ion is -1.arrow_forwardDraw the Lewis Structure & calculate the formal charge of the central atom.arrow_forwardPlease I really need help on #7Aarrow_forward
- Determine the formal charge for each element in the ammonium ion N = 0, H = +1 N = +5, H = 0 N = +1, H = -1 N = -3, H = +1 N = +1, H = 0arrow_forwardWhat is the Lewis Dot Structure and Formal Charge of the Oxygen atoms in these substances? (CH3)2O (CH3)3Oarrow_forwardConsider the incomplete structure shown. Determine the formal charge on the bromine atom in the structure. If the atom is formally neutral, indicate a charge of zero.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning