
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
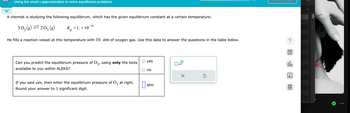
Transcribed Image Text:Using the small x approximation to solve equilibrium problems
A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature:
3 0₂ (g) = 203 (g)
K₂ = 1. × 10-6
P
He fills a reaction vessel at this temperature with 10. atm of oxygen gas. Use this data to answer the questions in the table below.
Can you predict the equilibrium pressure of 03, using only the tools
available to you within ALEKS?
If you said yes, then enter the equilibrium pressure of O3 at right.
Round your answer to 1 significant digit.
yes
no
atm
x10
X
Ś
olo
18
Ar
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 30, (g) = 20, (g) K, = 4. × 10-6 He fills a reaction vessel at this temperature with 9.0 atm of oxygen gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of O3, using only the tools Oyes available to you within ALEKS? O no O atm If you said yes, then enter the equilibrium pressure of O, at right. Round your answer to 1 significant digit.arrow_forwardBased on the formulas of the following solutes, which compound would have the smallest van't Hoff factor (i)? Ca(NO3)2(aq) K₂SO4(aq) Th(SO4)2(aq) Al₂(SO4)3(aq) MgSO4(aq)arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 30₂(g) = 203 (g) K₂ = 6.x 10-7 He fills a reaction vessel at this temperature with 13. atm of oxygen gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of O3, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of O3 at right. Round your answer to 1 significant digit. yes O no atm Xarrow_forward
- A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 NO(g) + Cl₂(g) 2 NOC1 (g) K₂ = 1. x 10 -6 He fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 17. atm of chlorine gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of NOCI, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of NOCI at right. Round your answer to 1 significant digit. O yes O no atm 0 X S BEEN 000 Ararrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2NO(g) + Cl2(g)⇌ 2NOCl(g) Kp=2.x10^−1 He fills a reaction vessel at this temperature with 10.atm of nitrogen monoxide gas and 12.atm of chlorine gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of NOCl, yes or no? If yes, then what is the equilibrium pressure of NOCl? Round your answer to 1 significant digit.arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 302(g)=20, (g) K = 1.x 10-2 He fills a reaction vessel at this temperature with 9.0 atm of oxygen gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of O3, using only the tools yes available to you within ALEKS? If you said yes, then enter the equilibrium pressure of O, at right. Round your answer to 1 significant digit. O no X Oatmarrow_forward
- A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH₂(g) C₂H₂(g) + 3H₂(g) K₂=2.x10-5 P He fills a reaction vessel at this temperature with 15. atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of C₂H₂, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of C₂H₂ at right. Round your answer to 1 significant digit. yes no atm ☐ x10 X ?arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 302(g)=203(g) -2 Kp =4. x 10 He fills a reaction vessel at this temperature with 15, atm of oxygen gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of O3, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of 03 at right. Round your answer to 1 significant digit. yes ☐ x10 O no X ☐ atmarrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH4 (g) 3H₂(g) + C₂H₂ (g) K₂=9. × 10-10 He fills a reaction vessel at this temperature with 5.0 atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of H₂, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of H₂ at right. Round your answer to 1 significant digit. yes no atm X S ?arrow_forward
- A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 6. N,(g) + 2H,0(g) 2 NO(g) + 2 H, (g) K,=7. × 10 He fills a reaction vessel at this temperature with 9.0 atm of nitrogen gas and 4.7 atm of water vapor. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of H,, using only the tools yes x10 available to you within ALEKS? no If you said yes, then enter the equilibrium pressure of H, at right. atm Round your answer to 1 significant digit.arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH₂(g)3H₂(g) + C₂H₂ (g) K₂=6. x 108 He fills a reaction vessel at this temperature with 11. atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of H₂, using only the tools available to you within ALEKS? If you said yes, then enter the equilibrium pressure of H₂ at right. Round your answer to 1 significant digit. yes no at atm x10 Xarrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: N2(g) + 2H2O(g) 2 NO(g) + 2 H2(g) K=2.x 10 -10 He fills a reaction vessel at this temperature with 6.0 atm of nitrogen gas and 11. atm of water vapor. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of H2, using only the tools available to you within ALEKS? ○ yes x10 O no If you said yes, then enter the equilibrium pressure of H₂ at right. Round your answer to 1 significant digit. atm 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY