
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
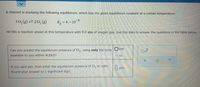
Transcribed Image Text:A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature:
30, (g) = 20, (g)
K, = 4. × 10-6
He fills a reaction vessel at this temperature with 9.0 atm of oxygen gas. Use this data to answer the questions in the table below.
Can you predict the equilibrium pressure of O3, using only the tools Oyes
available to you within ALEKS?
O no
O atm
If you said yes, then enter the equilibrium pressure of O, at right.
Round your answer to 1 significant digit.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s), to chlorine and iodine gases. The partial pressure of chlorine gas at equilibrium is three times that of iodine gas. What are the partial pressures of iodine and chlorine at equilibrium?arrow_forward. What does it mean to say that a state of chemical or physical equilibrium is dynamic?arrow_forwardThe following reaction is earned out at 500 K in a container equipped with a movable piston. A(g)+B(g)C(g);Kc=10(at500K) After the reaction has reached equilibrium, the container has the composition depicted here. Suppose the container volume is doubled. a How does the equilibrium composition shift? b How does the concentration of each of the reactants and the product change? (That is, does the concentration increase, decrease, or stay the same?)arrow_forward
- How does equilibrium represent the balancing of opposing processes? Give an example of an “equilibrium” encountered in everyday life, showing how the processes involved oppose each other.arrow_forwardMethanol, a common laboratory solvent, poses a threat of blindness or death if consumed in sufficient amounts. Once in the body, the substance is oxidized to produce formaldehyde (embalming fluid) and eventually formic acid. Both of these substances are also toxic in varying levels. The equilibrium between methanol and formaldehyde can be described as follows: CH3OH(aq)H2CO(aq)+H2(aq) Assuming the value of K for this reaction is 3.7 1010, what are the equilibrium concentrations of each species if you start with a 1.24 M solution of methanol? What will happen to the concentration of methanol as the formaldehyde is further converted to formic acid?arrow_forward. For an equilibrium involving gaseous substances, what effect, in general terms, is realized when the volume of the system is decreased?arrow_forward
- Consider the following equilibrium: COBr2(g) CO(g) + Br2(g)Kc = 0.190 at 73 C (a) A 0.50 mol sample of COBr2 is transferred to a 9.50-L flask and heated until equilibrium is attained. Calculate the equilibrium concentrations of each species. (b) The volume of the container is decreased to 4.5 L and the system allowed to return to equilibrium. Calculate the new equilibrium concentrations. (Hint: The calculation will be easier if you view this as a new problem with 0.5 mol of COBr2 transferred to a 4.5-L flask.) (c) What is the effect of decreasing the container volume from 9.50 L to 4.50 L?arrow_forward. Hydrogen gas, oxygen gas, and water vapor are in equilibrium in a closed container. Hydrogen gas is injected into the container, and the system is allowed to return to equilibrium. Which of the following occurs? Explain your answer. 2H2(g)+O2(g)2H2O(g)a. The concentration of oxygen gas remains constant. b. The value for K increases. c. The concentration of oxygen gas increases. d. The concentration of water vapor increases. e. The value for K decreases.arrow_forwardDuring an experiment with the Haber process, a researcher put 1 mol N2 and 1 mol H2 into a reaction vessel to observe the equilibrium formation of ammonia, NH3. N2(g)+3H2(g)2NH3(g) When these reactants come to equilibrium, assume that x mol H2 react. How many moles of ammonia form?arrow_forward
- 12.101 An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to capture toxic hydrogen sulfide gases present by reaction with aqueous iron(II) nitrate to form solid iron(II) sulfide. (a) Write the chemical equation for this process, assuming that it reaches equilibrium. (b) What is the equilibrium constant expression for this system? (c) How can the process be manipulated so that it does not reach equilibrium, allowing the continuous removal of hydrogen sulfide?arrow_forwardAt 2300 K the equilibrium constant for the formation of NO(g) is 1.7 103. N2(g) + O2(g) 2 NO(g) (a) Analysis shows that the concentrations of N2 and O2 are both 0.25 M, and that of NO is 0.0042 M under certain conditions. Is the system at equilibrium? (b) If the system is not at equilibrium, in which direction does the reaction proceed? (c) When the system is at equilibrium, what are the equilibrium concentrations?arrow_forwardA sample of gaseous nitrosyl bromide (NOBr) was placed in a container tiued with a frictionless, massless piston, where it decomposed at 25C according to the following equation: 2NOBr(g)2NO(g)+Br2(g) The initial density of the system was recorded as 4.495 g/L. After equilibrium was reached, the density was noted to be 4.086 g/L. a. Determine the value of the equilibrium constant K for the reaction. b. If Ar(g) is added to the system at equilibrium at constant temperature, what will happen to the equilibrium position? What happens to the value of K? Explain each answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning