
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
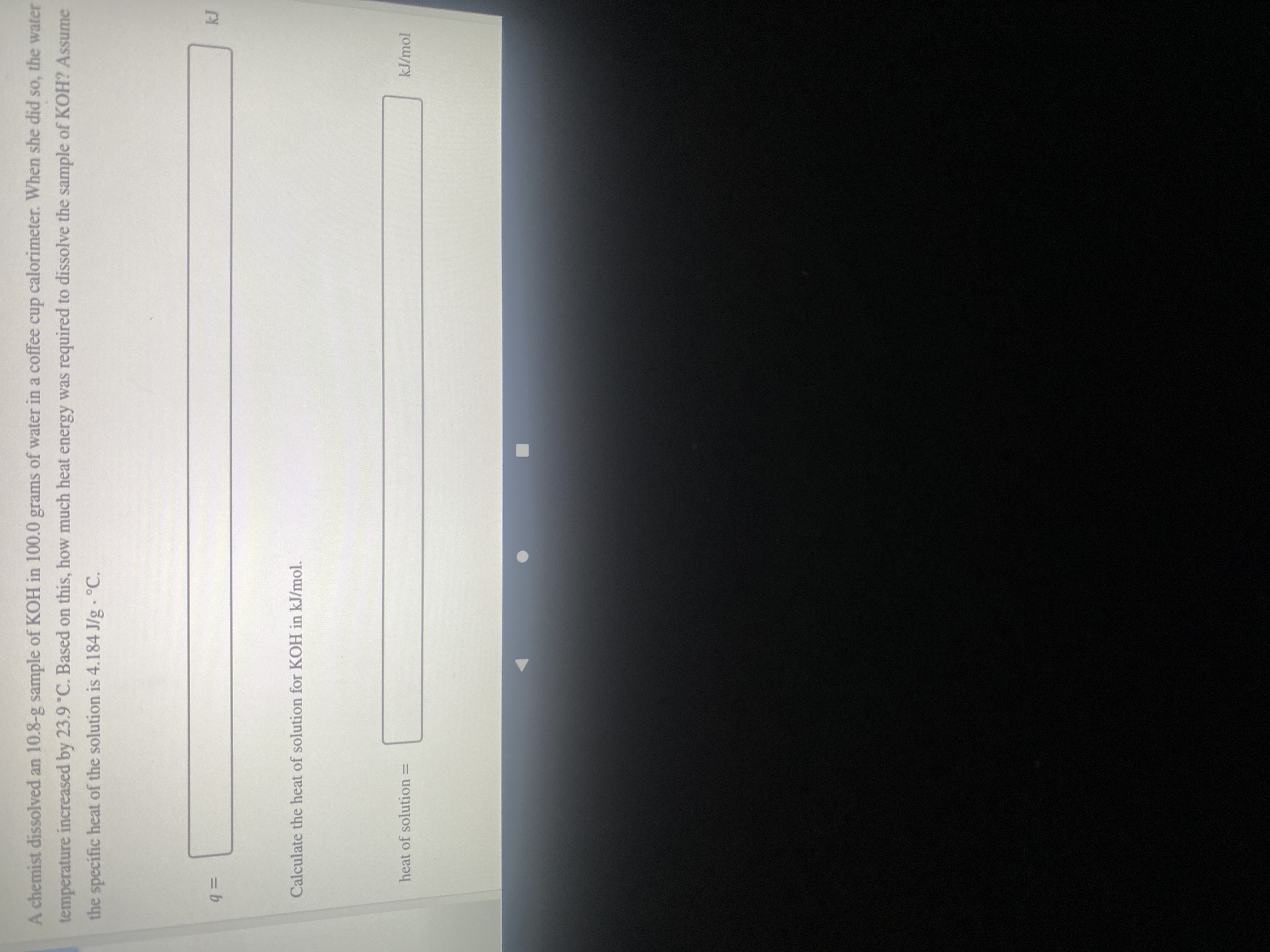
Transcribed Image Text:A chemist dissolved an 10.8-g sample of KOH in 100.0 grams of water in a coffee cup calorimeter. When she did so, the water
temperature increased by 23.9°C. Based on this, how much heat energy was required to dissolve the sample of KOH? Assume
the specific heat of the solution is 4.184 J/g - °C.
%3D
=Db
Calculate the heat of solution for KOH in kJ/mol.
kJ/mol
heat of solution =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature of an object increases by 39.4 °C when it absorbs 3715 J of heat. Calculate the heat capacity of the object. %3D J/°C The mass of the object is 393 g. Use the table of specific heat values to identify the composition of the object. Substance Specific heat (J/(g °C)) 0.129 silver 0.240 iron 0.444 aluminum 006'0 The object is composed of gold. aluminum.arrow_forward50.0 mL of hot water (49.6 oC) is added to 50.0 mL of cold water (24.7 oC). Upon mixing the water reaches an equilibrium temperature of 36.3 oC. Calculate the heat absorbed by the calorimeter in units of kJ/oC. Heat capacity for water is 4.184 J/(g·oC). -0.0267 kJ/oC -0.383 kJ/oC 0.356 kJ/oC -0.449 kJ/oC -0.0307 kJ/oCarrow_forward15.0 mL of a strong base is added to 15.0 mL of a strong acid in a coffee cup calorimeter. The temperature of the calorimeter contents rises from 22.5 °C to 24.5 °C. Assume that: concentration of base = 0.500 M concentration of acid = 0.500 M the specific heat of the solution is 4.184 J/g°C the density of the solution is 1.00 g/mL the coffee cup does not absorb or release energy itself Calculate the ΔH for this neutralization reaction in kJ per mole of base. Answer Options: A. –0.299 kJ/mol B. –33.5 kJ/mol C. –16.7 kJ/mol D. –0.502 kJ/molarrow_forward
- 150. g of water (Cs = 4.184 J/g°C) at 20.0 °C is added to a coffee cup calorimeter. A 35.0 g sample of silver (Cs = 0.129 J/g°C) is then placed into the water. At thermal equilibrium, the temperature of the calorimeter is 22.07 °C. What was the initial temperature of the silver sample?arrow_forward15.0 mL of a strong base is added to 15.0 mL of a strong acid in a coffee cup calorimeter. The temperature of the calorimeter contents rises from 22.5 °C to 24.5 °C. Assume that: concentration of base = 0.500 M concentration of acid = 0.500 M the specific heat of the solution is 4.184 J/g°C the density of the solution is 1.00 g/mL the coffee cup does not absorb or release energy itself Calculate the ΔH for this neutralization reaction in kJ per mole of base. Group of answer choices –0.299 kJ/mol –0.502 kJ/mol –16.7 kJ/mol –33.5 kJ/molarrow_forwardYou add some of the white crystals to a small test tube filled with water. The water in the test tube was initially at room temperature (25˚C). Once the crystals were added and the tube was stirred, the temperature of the solution decreased to about 15˚C and all of the crystals dissolved. what is the change in enthalpy (∆H) for this process?arrow_forward
- 67.0 mL of hot water (45.4 oC) is added to 45.0 mL of cold water (22.3 oC). Upon mixing the water reaches an equilibrium temperature of 35.1 oC. Calculate the heat absorbed by the calorimeter in units of kJ/oC. Heat capacity for water is 4.184 J/(g·oC). A-0.514 kJ/oC B-0.0463 kJ/oC C-0.0373 kJ/oC D-0.477 kJ/oC E-0.414 kJ/oCarrow_forwardWhen 6.72 grams of potassium chlorate (KCIO3) are dissolved in 120.0 grams of water at 25.0 °C in an insulated container, the temperature of the water decreases to 20.7 °C. Assuming that the specific heat of the solution is 4.184 J/(g °C) and that no heat is gained or lost by the container, what is the AH of solution of KCIO3 in kJ/mol?arrow_forwardA student mixes 67.0 mL of a 2.01 M sodium hydroxide solution with 22.4 mL of 6.45 M hydrochloric acid. The temperature of the mixture rises 17.2 ° C. The density of the resulting solution is 1.00 g mL and has a specific heat capacity of 4.184 J g · ° C . The heat capacity of the calorimeter is 16.97 J ° C . Part 1: (a) Identify the limiting reagent for the reaction. Part 2: (b) Calculate the heat of reaction (in J). qrxn = × 10 JEnter your answer in scientific notation. Part 3 out of 3 (c) Find the enthalpy of neutralization (in kJ/mol). ΔHneutralization = ____ kj/molarrow_forward
- A chemist dissolved an 11.4-g sample of KOH in 100.0 grams of water in a coffee cup calorimeter. When she did so, the water temperature increased by 25.1 "C. Based on this, how much heat energy was required to dissolve the sample of KOH? Assume the specific heat of the solution is 4.184 J/g °C. . Greaction= 4 Calculate the heat of solution (AH) for KOH in kJ/mol. heat of solution= kJ/molarrow_forwardWhen 12.29 g of finely divided brass at 95.0 °C is quickly stirred into 40.00 g of water at 22.0 °C, the water temperature rises to 24.0 °C. Assuming heat can only transfer between the brass and the water, calculate the specific heat of brass. (Specific heat capacity of water is 4.18 J/g* oC)arrow_forward"When 37.00 grams of fictional compound X is added to water to make 526.0 grams of solution, the temperature increases from 23.32 °C to 73.30 °C. What is the heat of solution for compound X given that the molar mass of compound X is 61.21 g/mol. Assume the specific heat capacity of the solution is 4.18 J/g°C."arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY