
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
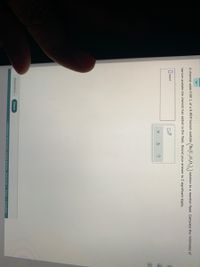
Transcribed Image Text:A chemist adds 0.80 L of a 0.48M barium acetate
solution to a reaction flask, Calculate the millimoles of
barium acetate the chemist has added to the flask. Round your answer to 2 significant digits.
mmol
Explanation
Check
2021 McGrawHi Education All Rights Resserved Terms of UseI PivacyI Accessibility
Expert Solution
arrow_forward
Step 1
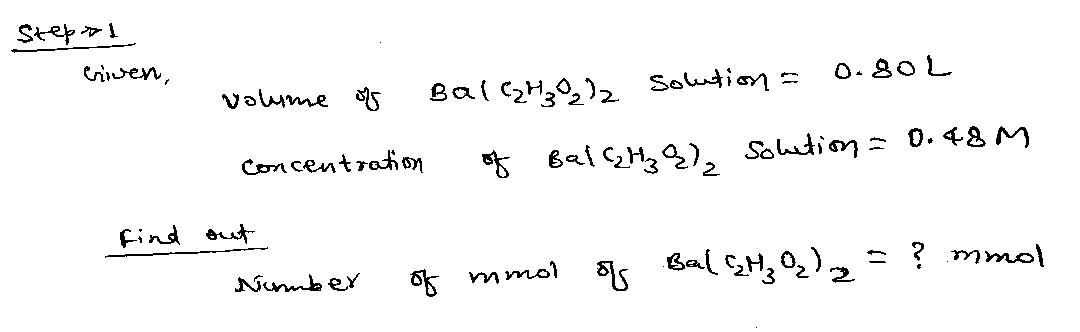
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Table 4. Concentration calculations Mass of empty evaporating dish (g) 29.258 Volume of NaCl solution (mL) 10.0 Mass of dish and NaCl solution (g) Mass of dish and dry NaCl (g) Mass of NaCl solution (g) Mass of dry NaCl (g) Mass/mass percent (%) Mass/volume percent (%) Moles of NaCl (mol) Volume of NaCl solution in liters (L) Molarity of NaCl solution (mol/L) 40.498 31.164arrow_forwardA hydrochloric acid solution will neutralize a sodium hydroxide solution. Part A Write a balanced chemical equation for the neutralization reaction. Express your answer as a chemical equation including phases. 0 - xa ΑΣΦ Submit Xb a b 016 a b A chemical reaction does not occur for this question. 18 Previous Answers Request Answer X Incorrect; Try Again X ? Review I Constants eriodic Tablearrow_forwardA chemist adds 1.70 L of a 0.39 mol/L barium chloride (BaCl) solution to a reaction flask. Calculate the millimoles of barium chloride the chemist has added to the flask. Round your answer to 2 significant digits. manol O.P Xarrow_forward
- A chemist adds 350.0mL of a 1.96M iron(II) bromide FeBr2 solution to a reaction flask. Calculate the millimoles of iron(II) bromide the chemist has added to the flask. Round your answer to 3 significant digits.arrow_forwardO CHEMICAL REACTIONS Solving limiting reactant problems in solution Suppose 1.75 g of potassium nitrate is dissolved in 250. mL of a 48.0 m Maqueous solution of sodium chromate. Calculate the final molarity of nitrate anion in the solution. You can assume the volume of the solution doesn't change when the potassium nitrate is dissolved in it. Be sure your answer has the correct number of significant digits. OM x10 3/5 Xarrow_forwardA reaction is made up in the following way: 16 mL of 4.3 M acetone + 16 mL of 1.1 M HCI + 16 mL of 0.0058 M I2 + 14 mL of water. What was the inital concentration of I2 in the reaction mixture? Express your answer as a decimal number (no exponents). Please include proper (abbreviated) units.arrow_forward
- A chemist adds 455.0 ml. of a 3.4M silver nitrate (AgNO, solution to a reaction flask. Calculate the millimoles of silver nitrate the chemist has added to the flask. Round your answer to 2 significant digits. Immolarrow_forwardI cannot understnad this question because the question is asking the ‘concentration’ but the solution showing just ‘number of moles’. Did I misunderstnading??arrow_forwardGive detailed Solution..don't give Handwritten answerarrow_forward
- Data Analysis and Interpretation 1. Marble is calcium carbonate, CaCO3. Its reaction with hydrochloric acid is CaCO3(s)+ 2HC1(aq) → CaCl2(?)+ CO2(g) + H2O(!) What was the gas produced when marble reacted with HC1? Using the solubility rules, determine the physical state for CaCl2, explain which rule you used. id Do thesearrow_forwardWhat volume (in mL) of 0.150 M HCI would be required to completely react with 4.30 g of Al in the following chemical reaction? 2 Al(s) + 6 HCI(aq) → 2 AICI, (aq) + 3 H,(g) 1 4 7 +/- MacBook Pro 888 %23 $ % 3 4 7 1 Q W E R Fab K A F lock C V M control option command * Carrow_forwardThe lab requires a 3.00 M HCI solution. How many mL of a 12.00 M HCI solution are required to produce 200. mL of this 3.00 M HCI solution? Please provide your answer to 3 significant figures. Add your answerarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY