
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
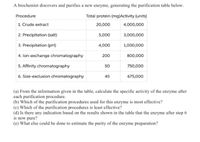
Transcribed Image Text:A biochemist discovers and purifies a new enzyme, generating the purification table below.
Procedure
Total protein (mg)Activity (units)
1. Crude extract
20,000
4,000,000
2. Precipitation (salt)
5,000
3,000,000
3. Precipitation (pH)
4,000
1,000,000
4. lon-exchange chromatography
200
800,000
5. Affinity chromatography
50
750,000
6. Size-exclusion chromatography
45
675,000
(a) From the information given in the table, caleulate the specific activity of the enzyme after
each purification procedure.
(b) Which of the purification procedures used for this enzyme is most effective?
(c) Which of the purification procedures is least effective?
(d) Is there any indication based on the results shown in the table that the enzyme after step 6
is now pure?
(e) What else could be done to estimate the purity of the enzyme preparation?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- A continuous enzyme reactor consists of a stirred tank of volume 0.01 m3 through which liquid is pumped at 0.02 m3⁄h. The enzyme is immobilised onto non-porous, spherical support particles, and the contents are stirred well to ensure that the composition is uniform and that the particles remain in suspension. A plastic mesh has been placed in front of the exit pipe to prevent the particles from leaving the vessel. The reactor is used to carried out a 98% conversion of a substrate entering at 0.1 kmol m3 ⁄ . Michaelis-Menten kinetics apply, with the following enzyme characteristics: Turnover number = 1 × 10^3 s KM = 5 × 10−3 kmol m3 ⁄ Enzyme RMM = 65,000 (a) If the enzyme is stable, calculate the mass of enzyme that needs to be immobilised. (b) If the enzyme is not stable and experiences exponential denaturation with a half-life of 10 h at the reaction temperature, determine the denaturation constant. (c) How would you expect denaturation to affect the reactor performance?arrow_forwardThis is my data collected from a Succinate Dehyrogenase Enzyme Assay - I am asked to plot a Concentration vs Time while plotting the Resuspended and Supernatant Reaction; how can this be completed? Test Tube Concentration (uM) Absorbance @ 600nm #1 0 0.00 #2 5 0.090 #3 10 0.187 #4 20 0.368 #5 40 0.715 #6 60 1.075 Table 3. Succinate Dehydrogenase Assay @ 600 nm Time point (min): Resuspended Pellet Reaction Absorbance Supernatant Reaction Absorbance Enzyme Reaction Control Absorbance 0 43.2 44.2 46.1 3 43.3 44.8 6 43.0 44.9 9 42.6 45.2 12 43.5 45.6 15 44.0 45.7 18 43.8 45.9 21 44.0 46.4 24 44.1 46.5 27 43.9 46.7 30 43.7 46.9 43.6arrow_forwardis there a role for the protein data bank in biochemical research? if so, describe it. I am so confused on what he is asking our textbook doesn't talk about that. we are using the principle of biochemistry eighth edition: David L.Nelsonarrow_forward
- For the biochemical tests, write in the name of the tests in order that you get them . Record your appearances and observations. Then indicate the result of the test.arrow_forwardBriefly describe how you would make 500 mls of a 1X electrophoresis running buffer from a 15X stock solution.arrow_forwardIn quantitative determination of protein using spectrophotometer how can I plot the absorbance vs wavelength for the proteins given the transmittance values?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON