
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
helpp pls
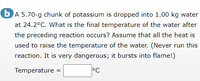
Transcribed Image Text:bA 5.70-g chunk of potassium is dropped into 1.00 kg water
at 24.2°C. What is the final temperature of the water after
the preceding reaction occurs? Assume that all the heat is
used to raise the temperature of the water. (Never run this
reaction. It is very dangerous; it bursts into flame!)
Temperature
°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Match the following amino acids with their chemical properties H -N-CIC- A -A ✓ B но с D VE ✓ F ✓ G / CH3 CH3 CH B H но -N-C-C- feed C но -N-C-C- H CH₂ D HO -N-CIC- 1 H CH₂ OH E но ||| -N-C-C- II H CH₂ I CH₂ CH₂ CH₂ T NH3 I. Acidic II. Nonpolar III. Polar uncharged IV, Basic + F O HO -N-C-C- H CH₂ NH₂ G но | || -N-C-C- I H CH₂ 1arrow_forwardKidney stones are crystals of calcium oxalate that form in the kidney, ureter, or bladder. Small kidney stones are passed out of the body easily, but larger kidney stones may block the ureter causing severe pain. If the [Ca2+] in blood plasma is 5 × 10-3 M, what [C2O42-] must be present to form a kidney stone? (Calcium oxalate Ksp = 2.3 x 10-9)arrow_forwardOnly typed solutionarrow_forward
- Is peptide bond 2 (colored red) drawn cis or trans? O trans O cis NH₂ SH NH 1 01 OH HN CH3 OHarrow_forwardTavie To.3 (Pages 550-550) in te texwook anu iuenuiy ie type of TIONCovalenIL interacuon expecieu Delweern uie side char acids. the appropriate items to their respective bins. Reset isoleucine and proline valine and phenylalanine glutamate and arginine glutamine and serine Hydrogen bonds Hydrophobic interactions Salt bridge vious 近arrow_forwardConsider the hexapeptide S-H-I-R-M-P Draw the structure at pH 1.0. What is the charge at this point? Draw the structure at pH 7.4. What is the charge at this point? Determine the pl and draw its structure.arrow_forward
- 1a. Describe the formation of a bond between two amino acids that craves a peptide. 1B. The image below is a dipeptide. How can you always identified the peptide bond between amino acids?arrow_forwardWhen patients are found to have increases or decreases in their total protein & albumin values, this test might be run next to narrow down the disease diagnosis: Question 19 options: serum protein electrophoresis A/G ratio glucose cholesterolarrow_forwardH₂N Combination of two or more protein units 7. Which type of cross-link involves interactions between two cysteine amino acids? 8. Which type of cross-link would form between the following amino acids? Η Ο || H₂N-C-C-OH T CH₂ C=O NH₂ Η Ο LL H₂N-C-C-OH T CH₂ OH 9. To what main enzyme class do the nzym that catalyze the following reaction belong? (oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases) HO | || I c-c-N-C-COO + H₂O IT HR₂ R₁ H 1 This bond is cleaved H H₂N-C-COOⓇ I R₂ Wilter navy q H I H₂N-C-C00 1 R₁ •Qisulfide 10. Briefly explain the Lock and Key enzyme model.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY