
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
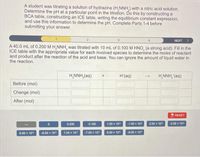
Transcribed Image Text:A student was titrating a solution of hydrazine (H,NNH,) with a nitric acid solution.
Determine the pH at a particular point in the titration. Do this by constructing a
BCA table, constructing an ICE table, writing the equilibrium constant expression,
and use this information to determine the pH. Complete Parts 1-4 before
submitting your answer.
NEXT >
A 40.0 mL of 0.200 M H,NNH, was titrated with 10 mL of 0.100 M HNO, (a strong acid). Fill in the
ICE table with the appropriate value for each involved species to determine the moles of reactant
and product after the reaction of the acid and base. You can ignore the amount of liquid water in
the reaction.
H,NNH,(aq)
H'(aq)
H,NNH, (aq)
Before (mol)
Change (mol)
After (mol)
S RESET
0.200
0.100
1.00 x 10
-1.00 x 10
2.00 x 10
-2.00 x 10°
7.00 x 10°
-7.00 x 10
8.00 x 10°
-8.00 x 10
6.00 x 10°
-6.00 x 10*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sketch the titration curve for a weak acid titrated by a strong base. When performing calculations concerning weak acidstrong base titrations, the general two-slep procedure is to solve a stoichiometry problem first, then to solve an equilibrium problem to determine the pH. What reaction takes place in the stoichiometry part of the problem? What is assumed about this reaction? At the various points in your titration curve, list the major species present after the strong base (NaOH, for example) reacts to completion with the weak acid, HA. What equilibrium problem would you solve at the various points in your titration curve to calculate the pH? Why is pH 7.0 at the equivalence point of a weak acid-strong base titration? Does the pH at the halfway point to equivalence have to be less than 7.0? What does the pH at the halfway point equal? Compare and contrast the titration curves for a strong acidstrong base titration and a weak acidstrong base titration.arrow_forwardA solution is prepared that is initially 0.078M in propanoic acid (HC,H,CO,) and 0.38M in potassium propanoate (KC,H,CO,). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in H30" |. You can leave out the M symbol for molarity. [HC,H,Co,] [c,,co] [1,0] initial change finalarrow_forwardA solution is prepared that is initially 0.27 M in benzoic acid (HC H CO,) and 0.11 M in sodium benzoate (NaC Hco.). Complete the reaction table below, so that you could use it to calculate the pH of this solution. [1,0"] Use x to stand for the unknown change in You can leave out the M symbol for molarity. [HC,H,CO.] [4,0] initial change final Explanation Check 0VI 10:3 acer DIl %23 elete 13 5 6 69 le tarrow_forward
- A solution is prepared that is initially 0.30M in hydrofluoric acid (HF) and 0.13M in potassium fluoride (KF). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in [H3O+]. You can leave out the M symbol for molarity. [HF] [F] [H,O] initial 0 0 change 0 final 0 0 ㅁ 1 Garrow_forwardPlease don't provide handwritten solution ....arrow_forwardDetermine the pH of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. NEXT > The buffer was prepared by dissolving 21.5 g HC,H,O₂ and 37.7 g of NaC,H,O₂ in 200.0 mL of solution. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) 0.178 0.880-x [0] [0.888] [1.31 + x) 0 0 0.888 1.31 + x HC₂H5O₂(aq) + [21.5] [x] Ka = 4.2 x 10-² 21.5 0.178 + x +x [37.7] [2x] 1 [0.178+x][0.888 + x] 37.7 -x 0.888 + x Determine the pH of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. 6.23 The Ka for HC₂H5O₂ is 6.3 x 10-5. Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka…arrow_forward
- Determine the pH of a solution by constructing a BCA table, constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. The value of Kb for NH3 is 1.8 x 10-5. Complete Parts 1-4 before submitting your answer. 1 2 3 4 NEXT Two solutions are mixed: 40.0 mL of 0.500 M NH3 and 25.0 mL of 0.300 M HCI. Fill in the table with the appropriate value for each involved species to determine the moles of reactant and product after the reaction of the acid and base. Before (mol) Change (mol) After (mol) NH3(aq) + H*(aq) -> NH, (aq) RESET - 0 40.0 0.500 25.0 0.300 0.300-x 0.0075 -0.0075 0.0100 -0.0100 0.0125 -0.0125 0.0200 -0.0200arrow_forwardA solution is prepared that is initially 0.26M in hydrofluoric acid (HF) and 0.25M in potassium fluoride (KF). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in [H₂O*]. You can leave out the M symbol for molarity. initial change final [HF] 0 П [F] 0 0 0 [H₂O*] 0 0 0 Xarrow_forwardA student was titrating a solution of acetic acid with a sodium hydroxide solution. Determine the pH at the equivalence point. Do this by constructing a BCA table, constructing an lCE table, writing the equilibrium constant expression, and use this information to determine the pH. Complete Parts 1-4 before submitting your answer. 3 NEXT > A 50 mL solution of 0.300 M CH COOH was titrated with 0.300 M NaOH. Fill in the table with the appropriate value for each involved species to determine the moles of reactant and product after the reaction of the acid and base. You can ignore the amount of water in the reaction.. CH,COOH(aq) + OH (aq) H,O(1) + CH,COO (aq) Before (mol) Change (mol) After (mol) RESET 0.300 -0.300 15.0 -15.0 ০.০300 -0.0300 0.150 -0.150 0.0150 -0.0150 50.0 -50.0arrow_forward
- Consider the titration of 100.0 mL of 0.100 M NaOH with 1.00 M HBr. Find the pH at the following volumes of acid added. See the picture below. Kindly answer all the questions, thanks!arrow_forwardWhat are the factors that affect buffer capacity? Explain in 2-3 sentences.arrow_forwardA solution is prepared that is initially 0.45M in pyridine (CÂHÂN), a weak base, and 0.50M in pyridinium bromide (C5H₁NHBr). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in [où¯]. You can leave out the symbol for molarity. initial change final [C,H,N] [C₂H₂NH*] [OH-] 0 0 0 0 DO X Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
