
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
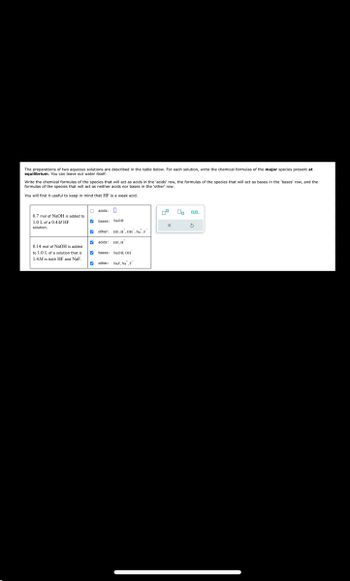
Transcribed Image Text:The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
0.7 mol of NaOH is added to
1.0 L of a 0.4M HF
solution.
acids:
bases: NaOH
X
0.14 mol of NaOH is added
to 1.0 L of a solution that is
1.4M in both HF and NaF.
other: HF.H.OH. Na, F
acids: HF,H
bases: NaOH, OH
other: NaF, Na, F
![A solution is prepared that is initially 0.30M in hydrofluoric acid (HF) and 0.13M in potassium fluoride (KF). Complete the reaction table below, so that you
could use it to calculate the pH of this solution.
Use x to stand for the unknown change in [H3O+]. You can leave out the M symbol for molarity.
[HF]
[F]
[H,O]
initial
0
0
change
0
final
0
0
ㅁ
1
G](https://content.bartleby.com/qna-images/question/7bc30bfd-54a4-45be-8be6-a7bac17a47af/16573e8f-6fc8-48f0-814e-70e032003d60/w7mcln_thumbnail.jpeg)
Transcribed Image Text:A solution is prepared that is initially 0.30M in hydrofluoric acid (HF) and 0.13M in potassium fluoride (KF). Complete the reaction table below, so that you
could use it to calculate the pH of this solution.
Use x to stand for the unknown change in [H3O+]. You can leave out the M symbol for molarity.
[HF]
[F]
[H,O]
initial
0
0
change
0
final
0
0
ㅁ
1
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base.arrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find useful to keep in mind that HCN is a weak acid. 1.1 mol of KOH is added to 1.0 L of a 1.1 MHCN solution. 0.2 mol of HCl is added to 1.0 L of a solution that is 0.4M in both HCN and KCN. acids: □ bases: CN other: K acids: 0 bases: other: X Śarrow_forwardThe preparation of an aqueous solution is described in the table below. For this solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. D acids: ☐ 2 mol of NaOH is added to 1.0 L of a 0.8M HF 0 bases: ☐ solution. X other: 00arrow_forward
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 0.6 mol of HCl is added to 1.0 L of a 0.6M NH3 solution. 0.2 mol of NaOH is added to 1.0 L of a solution that is 0.7M in both NH3 and NH,C1. acids: 0 bases: other: acids: bases: other: X 0,0,...arrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 2.3 mol of HNO3 is added to 1.0 L of a 1.4M NH3 solution. 0.3 mol of KOH is added to 1.0 L of a solution that is 0.7M in both NH3 and NH₂Br. acids: □ bases: other: acids: bases: other: 0 X 0,0,... Śarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 0.36 mol of HNO3 is added to 1.0 L of a 1.4M NH3 solution. 0.07 mol of NaOH is added to 1.0 L of a solution that is 0.7M in both NH3 and NH,C1. 4 acids: bases: other: acids: bases: other: 0 X 0,0,... Ś ? 000 18 Ararrow_forward
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH3CO2 is a weak acid. acids: 0.2 mol of NaOH is added to 1.0 L of a 0.7MHCH3CO2 solution. bases: ☐ other: ☐ 0.42 mol of HNO3 is added acids: ☐ to 1.0 L of a solution that is 1.0M in both HCH3CO2 bases: ☐ and NaCH3CO2. other: 1arrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH3CO2 is a weak acid. 1.6 mol of NaOH is added to 1.0 L of a 1.0M HCH3CO₂ solution. 0.62 mol of HBr is added to 1.0 L of a solution that is 1.5M in both HCH3CO₂ and KCH3CO₂. 0 0 acids: 0 bases: other: acids: bases: other: X 00 0,0,... Subscriptarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 1 mol of KOH is added to 1.0 L of a 0.9M HF solution. 0.46 mol of KOH is added to 1.0 L of a solution that is 1.0M in both HF and NaF. 0 acids: bases: other: acids: bases: other: 0 X Śarrow_forward
- The preparation of an aqueous solution is described in the table below. For this solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH,CO, is a weak acid. acids: 0.9 mol of NaOH is added to 1.0 L of a 0.9M HCH3CO2 bases: solution. other:arrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 1.0 mol of HNO3 is added to 1.0 L of a 1.0M NH3 solution. 0.08 mol of HNO3 is added to 1.0 L of a solution that is 0.7M in both NH3 and NH Br. O acids: bases: other: acids: bases: other: X 0,0,... Sarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH3CO₂ is a weak acid. acids: 0.7 mol of NaOH is added to 1.0 L of a 0.4M HCH₂CO₂ bases: solution. other: 0.09 mol of HI is added to acids: 1.0 L of a solution that is 0.5M in both HCH₂CO₂ and KCH3CO2. ☐ bases: other: 0,0,...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY