
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:[ools Help
CHEM LMAO Flashcards | Quizl X
calorimeter bomb equation - GX
A https://ezto.mheducation.com/ext/map/index.html?_con=con&external browser=0&launchUrl=htt
Saved
3 attempts left
Check my work
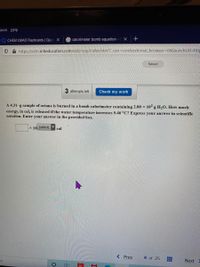
A 4.31-g sample of octane is burned in a bomb calorimeter containing 2.00 × 10² g H,O. How much
energy, in cal, is released if the water temperature increases 5.46 °C? Express your answer in scientific
notation. Enter your answer in the provided box.
x 10 (select)▼
cal
< Prev
4 of 25
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Historically, naphthalene was used as the main ingredient in mothballs and is easily recognized by its characteristic smell. When 0.500 g of naphthalene (C10H8) is burned in a bomb calorimeter, the temperature increased from 20.0 °C to 26.4 °C. The heat capacity of the calorimeter is 420 J/°C. Calculate the heat of combustion of naphthalene in kJ/mol.arrow_forwardA sample of 23.9 g of ammonium nitrate, NH,NO3, was dissolved in 133.8 g of water in a coffee cup calorimeter. The temperature changed from 28.10 °C to 17.42 °C. Calculate the heat of solution of ammonium nitrate in kJ/mol. Assume that the energy exchange involves only the solution and that the specific heat of the solution is 4.18 J/g°C. Heat of solution = i kJ/mol eTextbook and Media Save for Later Attempts: 0 of 2 used Submit Answer ype'here to search a 回D 中 ho 米 10 144 insert prt sc @ %23 %24 3 6. 7 8. backsp R Y A S D G H K pause C V B N ||M. alt ctrl altarrow_forwardCoal power plants burn large amounts of coal, C(s), in an O2(g) atmosphere to generate electricity. The chemical reaction responsible for producing this energy is shown below: C(s) + O2(g) → CO2(g) Determine the mass in grams of CO2 produced when 102 metric tons of C(s) are completely burned in an O2 atmosphere (1 metric ton = 103 kg = 106 g). Don't include units in your answerarrow_forward
- A 36.0−g sample of an unknown metal at 99 ° C was placed in a constant-pressure calorimeter containing 90.0 g of water at 24.0 ° C. The final temperature of the system was found to be 28.4 ° C. Calculate the specific heat of the metal. (The heat capacity of the calorimeter is 13.4 J/ ° C.)arrow_forwardIn a calorimetric analysis, 2.42 g of potassium chloride is weighed. The heat of solution of this salt is measured and calculated as 601 J. The molar heat of solution of potassium chloride is calculated. The actual value of the molar heat of solution of potassium chloride is 17.2 KJ/mol. Comparing with this actual value, what is the relative error? (Note: the molar mass of potassium chloride is 74.6 g/mol.)arrow_forwardMethyl alcohol (CH3OH) is also known as wood alcohol and can be used as fuel. When one mole of methyl alcohol is burned, 1453 kJ of heat are evolved. When methyl alcohol is burned in a bomb calorimeter, 71.8 kJ of heat are evolved by the methyl alcohol. If the density of methyl alcohol is 0.791 g/mL, find the volume of methyl alcohol burned.arrow_forward
- You just got a job teaching chemistry at a local community college. Your new boss wants you to devise a coffee cup calorimeter experiment that involves a modest temperature change of 4.00 Celsius, say from 21.00 Celsius to 25.00 Celsius. She tells you to dissolve solid calcium hydroxide in sufficient water to yield 85.0g solution. Assuming the water absorbs all of the heat from the resulting reaction, how many grams of calcium hydroxide should be used in this experiment to yield the desired temperature change?arrow_forwardTitanium reacts with iodine to form titanium(III) iodide, emitting heat, via the following reaction: 2Ti(s)+3I2(g)→2TiI3(s), ΔHrxn=−839kJ Determine the mass of titanium that reacts if 1.53×103 kJ of heat is emitted by the reaction. Express your answer to three significant figures and include the appropriate units. Determine the mass of iodine that reacts if 1.53×103 kJ of heat is emitted by the reaction. Express your answer to three significant figures and include the appropriate units.arrow_forwardA 6.55 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the temperature of the salt and water is 23.72°C. After the salt has completely dissolved, the temperature of the solution is 28.54°C. What is the total mass inside the calorimeter in grams?arrow_forward
- Nonearrow_forwardA student wishes to determine the heat capacity of a coffee-cup calorimeter. After she mixes 90.5 g of water at 67.5°C with 90.5 g of water, already in the calorimeter, at 19.1°C, the final temperature of the water is 35.0°C. Calculate the heat capacity of the calorimeter in J/K. Use 4.184 J/g°C as the specific heat of water. Enter your answer to three significant figures in unit of J/K.arrow_forwardIn a calorimetric analysis, 2.42 g of potassium chloride is weighed. The heat of solution of this salt is measured and calculated as 601 J. The molar heat of solution of potassium chloride is calculated. The actual value of the molar heat of solution of potassium chloride is 17.2 KJ/mol. Comparing with this actual value, what is the percent error? (Note: the molar mass of potassium chloride is 74.6 g/mol.) Select one: a. 8% b. 4% c. all options are incorrect d. 2%arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY