
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
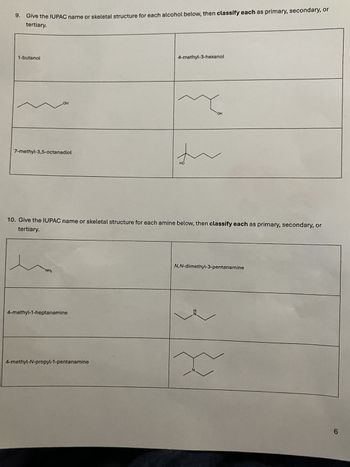
Transcribed Image Text:9. Give the IUPAC name or skeletal structure for each alcohol below, then classify each as primary, secondary, or
tertiary.
1-butanol
OH
7-methyl-3,5-octanediol
4-methyl-3-hexanol
OH
れ
HO
10. Give the IUPAC name or skeletal structure for each amine below, then classify each as primary, secondary, or
tertiary.
NH₂
4-methyl-1-heptanamine
4-methyl-N-propyl-1-pentanamine
N,N-dimethyl-3-pentanamine
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- A. Provide IUPAC names for the given structures or structures for the given names. N,4-dimethylpentane-2-amine 3-nitroaniline | ·N Br H₂N. CI-arrow_forwardidentify the functional group of each compound.arrow_forwardVancomycin is a useful antibiotic for treating infections in cancer patients on chemotherapy and renal patients on dialysis. How many amide functional groups are present in vancomycin? Which OH groups are bonded to sp3 hybridized carbon atoms and which are bonded to sp2 hybridized carbons?arrow_forward
- 0 0 carboxylic acid CH3CH,CCH2CH3 ester OCH2CH3 anhydride amide CH3CH2CH2CH2CH,COH none of the above CH,CH2CH2CH2CH2CNH2 Cengage Learning | Cengage Technical Support MacBook Airarrow_forwardWhat is the correct IUPAC name of the structure below?arrow_forwardIs the substituted group in NAG an amine or an amide? There is a carbon double bonded to an oxygen, and also attached to a nitrogen, however, the PPT says it is an amine.arrow_forward
- 15. It is believed that Alexander the Great was poisoned by an organic compound known as Strychnine. The structure of Strychnine is shown below. Identify the functional groups present in this molecule. a H "H b C --d A. a = tertiary amide, b = tertiary amine, c = alkene, d = ether B. a = ketone, b = secondary amine, c = alkene, d = ether C. a secondary amide, b = tertiary amine, c = alkyne, d = alcohol D. a = ketone, b = tertiary amide, c = alkyne, d = etherarrow_forwardLook at the structure of acetanilide (refer to the handout if the image doesn't show properly). Which functional groups are present in the compound (check all answers)? H. N' CH3 alkene aromatic alcohol ether alkyne amide ketone aldehyde amine alkane ester carboxylic acidarrow_forwardClassify each amine as 1˚, 2˚, or 3˚. Give the IUPAC name for each amine.arrow_forward
- 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol (bp 82°C), and 2-propanamine (bp 32°C) all have approximately the same molecular weight, yet their boiling points are quite different. Explain the reason for these differences.arrow_forward14-48 Explain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C), even though methanethiol has a higher molecular weightarrow_forward10-45 Draw structural formulas for the eight aldehydes with the molecular formula CgHi2O.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning