
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
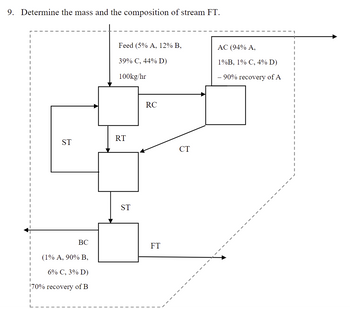
Transcribed Image Text:9. Determine the mass and the composition of stream FT.
Feed (5% A, 12% B,
39% C, 44% D)
100kg/hr
AC (94% A,
1%B, 1% C, 4% D)
- 90% recovery of A
RC
RT
ST
CT
ST
BC
FT
(1% A, 90% B,
6% C, 3% D)
70% recovery of B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Similar questions
- A mixture of benzene and toluene must be separated using a distillation column with the benzene purity at the top of the column as 92 % and bottom as 5%. The 250 kmol/h saturated liquid feed contains 55 mole% of benzene. The reflux is a saturated liquid and a reflux ratio of 1.9 is maintained. Estimate the distillate, bottom rate, minimum and actual number of stages required to obtain the desired purity. The vapor liquid equilibrium data at operating pressure of 101.3 kPa are given below. x|0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 y 0 0.21 0.38 0.511 0.627 0.719 0.79 0.853 | 0.91 0.961 | 1.0arrow_forwardShow all steps please, thanks.arrow_forwardChemical Engineering A flotation cell is fed with solids at the rate of 250tph with a pulp having a density of 1.35. Material solid density is 3.2. The concentrate contains 15% of the feed solids mass and it has a pulp density of 1.15. The concentrate solid density is 3.5. How much water must be added to the tails to give an L/S ratio of 1.25. Give your assumptions and continue answering.arrow_forward
- A stream containing propionic acid and water at 70:30 mass ratio is fed to a mixer at 3.181 kg/s with pure cyclohexane as solvent. What should be the flow rate of the solvent in order to attain 30:70 mass ratio of solute and solvent in the extract (solvent-rich) stream? Prepare graphical and computational solution. Propionic acid 0.0 1.0 0.1 0.9 0.2 0.8 0.3 0.7 +1 0.4 + ++ 0.6 0.5 0.5 0.6 0.4 0.7 0.3 0.8 0.2 0.9 0.1 1.0 0.0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Cyclohexane Waterarrow_forwardReverse Osmosis is used by water bottling companies to produce desalinized water from sea water. Giventhe figure below, determine the fraction of brine leaving the RO cell that is recycled.arrow_forwardExample 5.4. Redo the Example 5.2. once again, but consider the case that there is no recycle of the filtrate to be mixed with the fresh feed. Determine (a) the amount of K2Cro, produced per hour and (b) the rate that we need to evaporate water. Without the recycle of the filtrate, we can re-draw the flow chart as shown on the next page. We then try performing the balances around the whole process, as done previously. water vapour mz kg FIlter cake me kg K crystals (100%) makg 36.4% K 63.6% w 100% w Feed m -4.500 kg 33.3% makg 4941 50.6% w Crystalliser & Fater Evaporator Filtrate me kg 36.4%K 63.6% warrow_forward
- If the slurry is filtered using a larger filter press with an area of 15 m2 at a constant rate of 450 L/h, calculate the volume of filtrate that will be recovered and the time it will take to reach a pressure of 1000 kPa.arrow_forwardBackground (adapted from US EPA CERI-89-11) The operations at an industrial waste complex have contributed to a seven-acre hazardous waste disposal area located on the site. Figure 1 represents the general layout of the industrial complex. Site Description The site geological setting, as determined from existing surveys of the area is: Surface layer at the site is a sandy soil with a high permeability and a depth of 3 -5 ft. The subsurface has been characterized as a silty sandy clay that is moderately permeable and has a depth of approximately 30 feet. The depth to groundwater from the surface averages 30 feet across the site, and the depth to bedrock is approximately 65 feet. Groundwater flow is towards the east. The bedrock consists of an impermeable limestone. The climate in this area is very humid and has an average temperature of 72°F and an annual precipitation of 53.4 inches. The high and low temperatures in January are 74°F and 49°F and in August are 92 °F and 72°F,…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The