
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
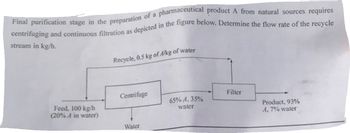
Transcribed Image Text:Final purification stage in the preparation of a pharmaceutical product A from natural sources requires
centrifuging and continuous filtration as depicted in the figure below. Determine the flow rate of the recycle
stream in kg/h.
Feed, 100 kg/h
(20% A in water)
Recycle, 0.5 kg of A/kg of water
Centrifuge
Water
65% A, 35%
water
Filter
Product, 93%
A, 7% water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- A wet paper pulp is found to contain 70% water. 61% of the original water was removed by drying. What is the concentration of water in the dried paper pulp? (use as the basis, 1 pound of wet pulp).arrow_forwardA double effect evaporator is used to concentrate an 18% CuSO4 solution. At the end of evaporation, 26,000 kg/h of 50% CuSO4 solution were obtained. If the water evaporated in the second effect is half the water evaporated in the first effect, determine: a) The amount of solution fed into the first effect. b) The amount of water evaporated in the first and second effect. c) The amount of solution fed into the second effect, as well as its composition. Problem diagram 18% CuSO4 82% H₂O M₁ M₂ M3 X CuSO4 Y H₂O A 2 M4 = 0.5 M₂ M5 H₂O steam M6 M2 M6 is the water that condenses 26 000 kg/hr 50% CuSO4 50% H2₂Oarrow_forwardAn evaporation-crystallization process is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 19.6% by mass K2SO4. The filter cake consists of solid K2SO4 crystals and a 40.0% by mass K2SO4 solution, in a ratio of 10 kg crystals/kg solution. The filtrate, also a 40.0% solution, is recycled to join the fresh feed. Of the water fed to the evaporator, 45.0% is evaporated. The evaporator has a maximum capacity of 175 kg of water evaporated per second.(a) Consider that the process operates at its maximum capacity. Draw and label the flow diagram and perform the degree of freedom analysis for the overall system, the fresh feed-recycle mixing point, the evaporator, and the crystallizer. Then, write in an efficient order (minimizing simultaneous equations) the equations you would use to solve for the unknowns. In each equation, indicate the variable to be determined, but do not perform the calculations yet.(b) Calculate the…arrow_forward
- Reverse Osmosis is used by water bottling companies to produce desalinized water from sea water. Giventhe figure below, determine the fraction of brine leaving the RO cell that is recycled.arrow_forwardcalculate the grams of sucrose that must be added to 415.9 grams of water to prepare a 16.01% by mass solutionarrow_forwardThe aqueous acetone solution is mixed with Methyl isobutyl (MIB) solvent containing 3% acetone by mass. The flow rates of the upper and lower currents leaving the system are 1000 kg/h and 400 kg/h, respectively. System the proportion of acetone in the upper and lower leaving current is 15% and 10% by weight, respectively. The phase separation of the refinate and the extract takes place. According to this a. Calculate the composition and amount of the aqueous acetone solution entering the system. b. Calculate the amount of Methyl isobutyl ketone (MIBK) solvent containing 3% acetone sent to the system. extract phase Data; Methyl isobutyl (MIB 0.05 0.05 0.06 0.07 0.08 0.09 0.20 XA Acetone 0 0.05 0.15 0.20 0.25 0.35 0.43 XC. Methyl isobutyl (MIB 0.95 0.94 0.89 0.84 0.79 Refining phase 0.69 0.59 YA Acetone 0 0.02 0.10 0.15 0.20 0.30 0.40 YCarrow_forward
- To make an instant tomato soup, a company makes soup powder from liquid step by a two-step process: concentrating the soup by removing water using a membrane to reach a concentration of 45% water, followed by a spray drying process which leads to a final moisture content of 3%. If the initial liquid tomato soup contains 9.0% solids and fat and enters the membrane separator at 4000 kg/hr, calculate: The flow-rate of the concentrated soup after membrane separation: kg/hr The flow-rate of water removed by the spray drying process (the second stage): kg/hrarrow_forwardIf the slurry is filtered using a larger filter press with an area of 15 m2 at a constant rate of 450 L/h, calculate the volume of filtrate that will be recovered and the time it will take to reach a pressure of 1000 kPa.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The