
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
pls help asap
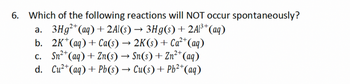
Transcribed Image Text:6. Which of the following reactions will NOT occur spontaneously?
->
2+
13+
a. 3Hg²+(aq) + 2Al(s) → 3Hg(s) + 2Al³+ (aq)
b. 2K+(aq) + Ca(s) → 2K(s) + Ca²+ (aq)
c. Sn2+(aq) + Zn(s) → Sn(s) + Zn2+(aq)
d. Cu2+(aq) + Pb(s) → Cu(s) + Pb2+(aq)
->
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forwardAccording to the Resource Conservation and Recovery Act (RCRA), waste material is classified as toxic and must be handled as hazardous if the lead concentration exceeds 5 mg/L. By adding chloride ion, the lead ion will precipitate as PbCl2, which can be separated from the liquid portion. Once the lead has been removed, the rest of the waste can be sent to a conventional waste treatment facility. How many grams of sodium chloride must be added to 500 L of a waste solution to reduce the concentration of the Pb2+ ion from 10 to 5 mg/L?arrow_forwardThe iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing small water-soluble ions and molecules. The iron in the aqueous solution is reduced to iron(II) ion and then titrated against potassium permanganate. In the titration, iron(ll) is oxidized to iron(III) and permanganate is reduced to manganese(II) ion. A 5.00-g sample of hemoglobin requires 32.3 mL of a 0.002100 M solution of potassium permanganate. The reaction with permanganate ion is MnO4(aq)+8H+(aq)+5Fe2+(aq)Mn2+(aq)+5Fe3+(aq)+4H2O What is the mass percent of iron in hemoglobin?arrow_forward
- One of the few industrial-scale processes that produce organic compounds electrochemically is used by the Monsanto Company to produce1,4-dicyanobutane. The reduction reaction is 2CH2CHCH+2H++2eNC(CH2)4CN The NC(CH2)4CN is then chemically reduced using hydrogen gas to H2N(CH2)6NH2, which is used in the production of nylon. What current must be used to produce 150.kg NC(CH2)4CN per hour?arrow_forwardWrite balanced net ionic equations for the following reactions in acid solution. (a) Liquid hydrazine reacts with an aqueous solution of sodium bromate. Nitrogen gas and bromide ions are formed. (b) Solid phosphorus (P4) reacts with an aqueous solution of nitrate to form nitrogen oxide gas and dihydrogen phosphate (H2PO4-) ions. (c) Aqueous solutions of potassium sulfite and potassium permanganate react. Sulfate and manganese(II) ions are formed.arrow_forwardWhat is Hrxn for reaction of iron(III) oxide and carbon monoxide to give iron metal and carbon dioxide gas? Use the following reactions: 4Fe(s)+3O2(g)2Fe2O3(s)H=1648.4kJ4CO(g)+O2(g)2CO3(g)H=565.98kJarrow_forward
- Bromine is obtained from sea water by the following redox reaction: Cl2(g) + 2 NaBr(aq) 2 NaCl(aq) + Br2() (a) What has been oxidized? What has been reduced? (b) Identify the oxidizing and reducing agents.arrow_forward1. Sometimes a reaction can fall in more than one category. Into what category (or categories) does the reaction of Ba(OH)2(aq) + H+PO4(aq) fit? acid-base and oxidation-reduction oxidation-reduction acid-base and precipitation precipitationarrow_forwardBalance each of the following equations, and classify them as precipitation, acid-base, gas-forming, or oxidation-reduction reactions. Show states for reactants and products (s, , g, aq). (a) CuCl2 + H2S CuS + HCl (b) H3PO4 + KOH H2O + K3PO4 (c) Ca +HBr H2 + CaBr2 (d) MgC12 + NaOH Mg(OH)2 + NaClarrow_forward
- An electrolytic cell is set up with Cd(s) in Cd(NO3)2(aq) and Zn(s) in Zn(NO3)2(aq). Initially both electrodesweigh 5.00 g. After running the cell for several hours theelectrode in the left compartment weighs 4.75 g. (a) Which electrode is in the left compartment? (b) Does the mass of the electrode in the right compartmentincrease, decrease, or stay the same? If the masschanges, what is the new mass? (c) Does the volume of the electrode in the right compartment increase, decrease, or stay the same? If the volumechanges, what is the new volume? (The density of Cd is8.65 g/cm3.)arrow_forwardWhat is the oxidation number of S in each of the following molecules? a. S2 b. S8 c. H2S d. SO3arrow_forward1. If you wish to convert 0.0100 mol of Au3+ (aq) ions into Au(s) in a “gold-plating” process, how long must you electrolyze a solution if the current passing through the circuit is 2.00 amps? 483 seconds 4.83 104 seconds 965 seconds 1450 secondsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
