
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
6
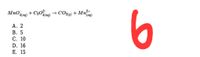
Transcribed Image Text:MnO (ag)
+ C20log) → CO2i) + Mn)
6
4(aq)
А. 2
В. 5
С. 10
D. 16
Е. 15

Transcribed Image Text:6. What is the coefficient of the species that will be in the greatest amount if there are 12.5 moles
of sodium oxalate reacted with 12.5 moles of potassium permanganate in the following reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- mistry F20 SE 1. If you have 23.2 grams of gold, what is the volume of the sample? Densities of Some Common Metals Metal Density (g/mL) Copper 8.94 the Aluminum 2.70 Gold 19.3 (A) 0.116 ml (B) 0.832 mL (C) 1.20 ml (D) 8.59 mL DELLarrow_forward8:41 AM Thu Mar 9 Items Back 22-23 Q3 SUM CHEMISTRY ADV Element Hydrogen Oxygen Sulfur A ALEKS - KEH... Molar mass 1.008 g/mol 15.999 g/mol 32.066 g/mol M Sign in to yo... Mach B Technologies Inc. © 2023 - gp.edugence.com ●●● What is the theoretical yield of sulfuric acid? Answer F) 613 g H₂SO4 G) 630 g H₂SO4 H) 651 g H₂SO4 J) 695 g H₂SO4 GP ISD Parents & St... 22-23 Q3 SUM CHEMISTRY ADV e gp.edugenc... SO3 + H₂O → H₂SO4 - x² X ◄▬ 6 500 g of sulfur trioxide react with excess water to produce sulfuric acid H₂SO4 in the reaction represented by the equation below. VPN + S G You find that... × × × × X X 10% X A 5arrow_forwardAq of NH4+ Aq of HF NH4+=6.25M HF=1.04x10^-6M NH3=5.00x10^-4M F=2.50x10^-5M H3O+=5.00x10^-4M H3O+=2.5x10^-5M What is the pH of both solutions? What is the concentration of OH- ions and the pOH of both solutions?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY