
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
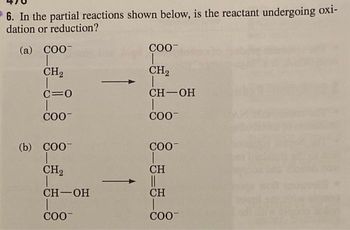
Transcribed Image Text:6. In the partial reactions shown below, is the reactant undergoing oxi-
dation or reduction?
(a) COO-
CH₂
T
C=O
COO™
(b) COO-
CH₂
CH-OH
T
COO-
COO™
CH₂
CH-OH
COO™
COO™
CH
CH
COO™
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. What is the major organic product generated in the reaction below? (A) CI OH (B) A.IOH C Cl₂ / H₂O (C) ||||C OH (D) OH CIarrow_forwardEnter the balanced equation for the ionization of the following carboxylic acid in water. Express your answer as a chemical equation without phases using the formula CH3,CH(CH3),COOH for the carboxylic acid.arrow_forwardUse curved arrows to show the flow of elections on the reactant side of each of the following reactionsarrow_forward
- i. Which of the reactions given below is wrong? H3O*/H20 (A) MgBr + CO2 H2/Pt (B) CH;CH2CN CH;CH,NH2 heat NABH4 (C) HO OH/H20 (D) CI OHarrow_forward9C Complete the figure below. J H₂ Lindlar catalyst Na H OH SOCI₂arrow_forwardIndicate whether the marked carbon atoms in the three molecules here are oxidized or reduced relative to the marked carbon atom in ethanol: There is no need to calculate oxidation states in this case; instead, just compare the types of atoms bonded to the marked carbon atoms:arrow_forward
- Which value (if any) corresponds to a faster reaction: (a) Ea = 40 kJ/mol or Ea = 4 kJ/mol; (b) a reaction temperature of 0 °C or a reaction temperature of 25 °C; (c) Keq = 10 or Keq = 100; (d) ΔH° = −10 kJ/mol or ΔH° = 10 kJ/mol?arrow_forwardWhich value (if any) corresponds to a faster reaction: (a) Ea = 40 kJ/mol or Ea = 4 kJ/mol; (b) a reaction temperature of 0 °C or a reaction temperature of 25 °C; (c) Keq = 10 or Keq = 100; (d) ΔHo = −10 kJ/mol or ΔHo = 10 kJ/mol?arrow_forwardWhich value (if any) corresponds to a faster reaction: (a) Ea = 40 kJ/mol or Ea = 4 kJ/mol;(b) a reaction temperature of 0 °C or a reaction temperature of 25 °C; (c) Keq = 10 or Keq = 100; (d) ΔHo = −10 kJ/mol or ΔHo = 10 kJ/mol?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co