
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
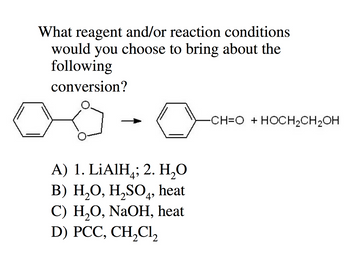
Transcribed Image Text:What reagent and/or reaction conditions
would you choose to bring about the
following
conversion?
C
A) 1. LiAlH₂; 2. H₂O
B) H₂O, H₂SO4, heat
C) H₂O, NaOH, heat
D) PCC, CH₂Cl₂
CH=O + HOCH₂CH₂OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The balanced chemical equation for the reaction between calcium hydroxide and hydrochloric acid is: Ca(OH)2 ( aq) + 2 HC1 ( aq )→ CaCl2 ( aq ) + 2 H,0 (1) We can interpret this to mean: 1 mole of calcium hydroxide and |mole(s) of hydrochloric acid React to produce: | mole(s) of calcium chloride and |mole(s) of waterarrow_forward4. An old antacid commercial claimed that each tablet of their product could "neutralize 47 times its mass in stomach acid". The active ingredient in the antiacid tablet, NaAl(OH)₂CO3, reacts with HCl in stomach acid according to this balanced reaction: NaAl(OH)₂CO3 + 4 HCl →→ NaCl + AlCl³ + 3 H₂O + CO₂ How many moles of HCl can a 1.03 g of antiacid tablet neutralize if the tablet contains 0.246 g of the active ingredient? If stomach acid has a concentration of 0.14 M HCl, assuming the density of stomach acid to be similar to that of water (1.00 g/mL), what is the mass of stomach acid that the 1.03 g of antiacid tablet can neutralize? Does this number support the claim in the commercial?arrow_forwardSolid copper can be produced by passing gaseous ammonia over solid copper (II) oxide at high temperatures, according to the following reaction. NH3 (g) + CuO (s) → N2 (g) + Cu (s) + H2O (g) Balance the reaction.arrow_forward
- Assuming you started with 3.23 g of sodium bicarbonate and your actual yield of solid product is 1.20g, which reaction is more likely to be the correct one?arrow_forward2. Balance the equation below for the reaction of benzil with sodium borohydride. ○ O C-C + BH4 + H₂O HH C-C- | | OH OH + 3. Calculate the number of moles of sodium borohydride and benzil used in the experiment. Considering the balanced equation, which of these is the limiting reactant? B(OH)4arrow_forwardWhat is the molarity of a hydrchloric acid, HCl, solution if it takes 26.90 mL of acid to neutralize 7.77 g of Fe(OH)3? The molar mass of Fe(OH)3 is 106.87 g/mol and the molar mass of HCl is 36.46 g/mol. The balanced chemical reaction is: 3 HCl (aq) + Fe(OH)3 (aq) → FeCl3 (aq) + 3 H2O (l)arrow_forward
- Assuming you started with 3.23 g of sodium bicarbonate and your actual yield of solid product is 1.20g, which reaction is more likely to be the correct one?arrow_forwardA titration is performed to determine the amount of sulfuric acid, H2SO4,, in a 6.5mL sample taken from a car battery. About 50mL of water is added to the sample, and then it is titrated with 43.37mL of standard 0.5824 molar NaOH solution. How many moles of sulfuric acid are present in the original sample?arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [1] → KC10(aq) + H₂O(1) 1-0arrow_forward
- It is known that when mixed in solution aqueous, silver ions will react with chloride ions to form insoluble silver chloride. Upon dissolving a 5.000 g solid mixture of AgNO3 and KNO3 in a concentrated KCl solution, 1.582 grams of a white precipitate, AgCl(s), formed. Determine the percent, by weight, of AgNO3 in the original mixture.arrow_forwardAccording to the following balanced reaction, how many moles of CaOCaO are required to exactly react with 6.55 moles of H2OH2O?CaOCaO(s) + H2OH2O(l) →→ Ca(OH)2Ca(OH)2(s)arrow_forwardcomplete the balanced molecular chemical equation for the reaction below. if no reaction occurs, write NR after the reaction arrow KOH(aq)+AlCl3(aq)=arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY