
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
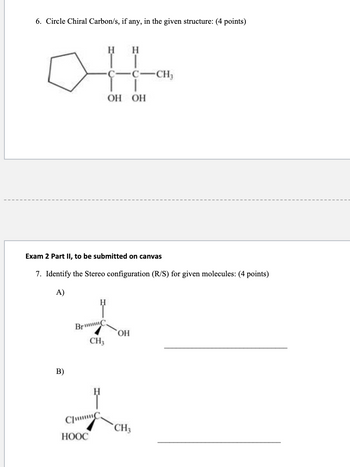
Transcribed Image Text:---
### Chemistry Practice Questions
#### 6. Circle Chiral Carbon/s, if any, in the given structure: (4 points)
**Structure:**
A structural diagram of a cyclopentane ring attached to a carbon chain. The carbon chain has two hydroxyl (OH) groups, and each carbon atom is bonded as follows:
- **First carbon (attached to cyclopentane):** Hydrogen (H), Hydroxyl (OH)
- **Second carbon:** Hydrogen (H), Hydroxyl (OH), Methyl group (CH₃)
---
#### Exam 2 Part II, to be submitted on Canvas
#### 7. Identify the Stereochemical Configuration (R/S) for Given Molecules: (4 points)
**A)**
- Diagram of a chiral center carbon bonded to four groups:
- Hydrogen (H)
- Bromine (Br)
- Hydroxyl (OH)
- Methyl group (CH₃)
*Answer field: ___________________________
**B)**
- Diagram of a chiral center carbon bonded to four groups:
- Hydrogen (H)
- Chlorine (Cl)
- Carboxyl group (HOOC)
- Methyl group (CH₃)
*Answer field: ___________________________
---
Expert Solution
arrow_forward
Step 1
Chiral carbon is carbon which contains all four different groups.Every chiral carbon has some configuration Either R or S .
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chair structure of a trisubstituted cyclohexane is shown below. Determine which of the following 2D representations matches the chair structure. CH3 CH3 H3C- CH3 A) I II CH3 CH3 CH3 B) I| CH C) II CH3 IV CH, CH3 CH3 D) IVarrow_forwardExpress each answer as a numerical digit, not as a word. How many sp-hybridized carbon atoms are in a molecule of 2-pentenal? How many sp2-hybridized carbon atoms are in a molecule of 2-pentenal? How many sp3-hybridized carbon atoms are in a molecule of 2-pentenal? How many sp3d-hybridized carbon atoms are in a molecule of 2-pentenal? How many sp3d2-hybridized carbon atoms are in a molecule of 2-pentenal?arrow_forwardDetermine absolute configuration (R or S)arrow_forward
- Is this right, can you check it pls. both are together/connectedarrow_forwardSelect the ring flip for the following compound: 1 • OH CI CI 이 OH H OH OHarrow_forwardFunctional Group Absorption (cm ¹) Alkane C-H 2850-2960 Alkane C-C Alkene =C-H Alkene C-C Alkene RCH-CH₂ 910 and 990 Alkene R₂C=CH₂ 890 Alkyne C-H Alkyne CEC он a H₂C-CH-CH₂ 800-1300 Submit Answer 3020-3100 1640-1680 3300 2100-2260 E Try Another Version Functional Group Absorption (cm³¹) Alkyl halide C-Cl Alkyl halide C-Bri a Alcohol O-H b Alcohol C-O Arene C-H and b CH₂CHCH₂CH₂ Aromatic ring C=C Amine N-H Amine C-N 600-800 tem attempts remaining 500-600 3400-3650 1050-1150 What IR absorption would you use to distinguish between the two compounds above? Enter a frequency, or range as lower freq-higher freq, from the table above. Which compound will exhibit this absorption? 3030 1660-2000 1450-1600 3300-3500 1030-1230 Functional Group Aldehyde C=O Ketone C=O Ester C=0 Amide C=O Carboxylic acid C=O Nitrile CEN Nitro NO₂ Absorption (cm ¹) cm 1 1730 1715 1735 1690 Carboxylic acid 0-H 2500-3100 1710 2210-2260 1540arrow_forward
- The skeletal structure in line-angle (line-bond) mode of 2,3,3,5-tetramethylhexane is shown. Identify the number of hydrogen atoms bound to each carbon atom in the structure. Answer Bank 3 2 1arrow_forwardCheck all of the compounds in the list below that are constitutional (structural) isomers of each other. О Н +н Н H Н Н H С. C H # -Н Н H Н Н Н Н Н- Н Н C Н Н Н Н te Н OH Н None of the Above Н H Н Harrow_forwardQ3) a) Which of the following compound are aromatic? (10 marks) Br H;C b) What is the hybridization of each of the carbon atoms in the following compound? 1) CH;CHCHC(CH3);CCH 2) CHCC(OH)2CH3 (10 marks) 3) Toluenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY