
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
a) What is the molecular formula of molecule A?
Write your formula in the format C#H#, where # stands for a number. If other atoms are present, write them in alphabetical order after hydrogen.
b) what is the IHD?
c) what
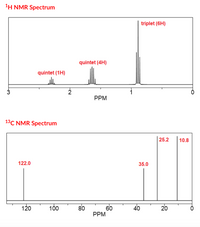
Transcribed Image Text:1H NMR Spectrum
triplet (6H)
quintet (4H)
quintet (1H)
PPM
13C NMR Spectrum
| 25.2
10.8
122.0
35.0
120
100
80
60
40
20
PPM
3.
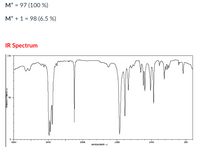
Transcribed Image Text:M* = 97 (100 %)
%3D
M* + 1 = 98 (6.5 %)
IR Spectrum
LOD
4000
3000
2000
1500
1000
500
HAVENUMB ERI -1l
TEHNSHLTTHNCET%T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- name all the functional groups of this molecule, there are 3arrow_forwardwhen looking at the name of a molecule the suffix tells you whether there are any double or helpful in answering questions on the subsequent pages. triple bonds in the molecule and the root tells you how many carbon atoms are present. Your instructor will guide you as you fill in the following table. The information in this table will be Note the suffix in each of the following functional groups: Alkanes have only ● • Alkynes have Alkenes have 1 Note the root associated with each of the following number of atoms: # of Carbons Example 2 bonds between carbon atoms. bonds between at least two of the carbon atoms. bonds between at least two of the carbon atoms. Root CH4 H Name this res charge! ~ 3 m ㅋ o bonds Can't be righ-arrow_forwardIdentify the selected functional groups. Type the name in the box below.arrow_forward
- What are the two functional groups? What is the parent chain? Why did you number the parent chain the way you did? What is the name of the molecule?arrow_forwardChoose all the functional groups that are found in this molecule: ОН ОН H. Holl H A carboxylic acid (B ketone c) ester (D alcohol (E) ether (F) aldehyde Ilarrow_forwardDraw a structural formula for 3-methylpentane. You do not have to draw H atomsarrow_forward
- Subject: chemistryarrow_forwardHow many carbons are present in the organic molecule below? a) 3 b) 4 c) 5 d) 6arrow_forward5. Circle and name the functional groups in the molecules below. Search the web where needed-you do not need to memorize these! Note: Atoms in conjugation are considered part of a single functional group. Each molecule has only one notable functional group. OH Brarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY